Digital therapeutics from bench to bedside

Definition of digital therapeutics
In 2015, Sepah et al. first mentioned the term “digital therapeutics” and expressed that DTx are “evidence-based behavioral treatments delivered online” that can increase healthcare accessibility and effectiveness5. The DTA, one of the most active organizations in defining and disseminating DTx, defined DTx as “evidence-based therapeutic interventions that are driven by high-quality software programs to treat, manage, or prevent a disease or disorder”6. However, no clear international agreement on the definition exists, and it is being interpreted or applied differently in countries and research institutes. When examining the definitions and responses to DTx in each country, South Korea is the only country that has defined DTx: “software as a medical device that provides evidence-based therapeutic intervention to patients to prevent, manage, or treat a medical disorder or disease”. In other countries, such as the US, Germany, the UK, Japan, Australia, China, and France, DTx are not defined at the government level and are treated as general medical devices, as summarized in Table 1.
The term DTx is similar to software as a medical device (SaMD) in that they both represent software used for medical purposes. While SaMD is a generic term for software intended to be used for one or more medical purposes, the term DTx is limited to software that intervenes with treatment based on clinical evidence for the treatment, management, or prevention of diseases or disorders. Therefore, most public, or regulatory authorities treat DTx as a sub-concept of SaMD without a special regulatory category.
Characteristics of digital therapeutics
Table 2 shows a comparison of the characteristics of pharmacotherapy and DTx. A common characteristic of conventional pharmacotherapy and DTx is that the therapeutic effect for a specific disease must be clinically verified, and a prescription is required; however, they have differences in all stages from development, clinical trial, regulatory approval, distribution, clinical application, and post-marketing management. DTx require minimal development cost compared with pharmaceuticals (<1%), and the development period is approximately half7. In addition, because they are not consumed due to use, manufacturing facilities and material costs for additional production after initial development are not required. Even for the distribution channels, unlike pharmaceuticals delivered to patients through manufacturers, wholesalers, retailers, and medical suppliers, DTx simplify the delivery path to patients through developers and platform providers.
For clinical trials, unlike pharmaceuticals that require phase-3 clinical trials that include drug safety and pharmacokinetic evaluation, DTx can obtain marketing approval through piloting (optional) and pivotal clinical trials through the medical device regulatory pathway in most countries8,9. Additionally, DTx only provide software-based interventions; thus, unlike existing pharmacotherapy that has side effects due to drug toxicity, they are considered to have only minor side effects owing to the use of mobile devices1. Medication adherence of DTx is known to be approximately 80%10, which is higher than that of pharmacotherapy (50%)11. In post-marketing management, the expiration date of pharmaceuticals is determined according to the denaturation of substances, but the expiration date of DTx depends on the efficacy that changes over time. In classification, pharmaceutical drugs are classified as over the counter or ethical the counter drugs depending on whether a prescription is required. Similarly, some of DTx are classified as a PDTx if it requires a prescription. Moreover, DTx can improve their function (efficacy) or discard it through updates. In pharmacotherapy, individual physiological characteristics are the most important influencing factors in determining drug efficacy. However, the important difference is that the efficacy of DTx can be affected not only by demographic factors but also by sociocultural and cognitive abilities12,13. The main disadvantages of DTx compared to pharmaceuticals are low patient accessibility from a digital barrier, need for prerequisites, and data security issues. Since DTx operate on a digital platform, it is essential to understand digital devices; therefore, education prior to use is required, and it can be applied only to patients who have digital devices and some level of cognitive ability. In addition, since DTx store user data in digital form, there is a risk of leakage of sensitive personal data; therefore, additional preventative cyber security is required. The clinical challenge of pharmacotherapy is considered as a blood–brain barrier in drugs for neurological disease; however, for DTx, patient engagement is considered a major factor in determining the success of treatment in the future as it presupposes active participation of the patient14.
Clinical trials of digital therapeutics
After screening 50,872 investigated clinical literature based on duplication, year of publication, non-journal article, and research scope, forty-five clinical trials were finally analyzed, excluding cases without National Clinical Trial (NCT) number or meta-analysis; of these, thirty-one were registered with clinicaltrials.gov and fourteen were presented in DTA website. A flowchart of the clinical trial search process is shown in Supplementary Figs. 1, 2, 3, and 4, and the NCT-numbered clinical trials related to DTx are summarized in Supplementary Table 1.
Detailed clinical trials related to psychiatric indications showed the largest number at 31.1%, neurological at 22.2%, endocrine at 20%, respiratory at 11.2%, poisoning at 8.9%, and cardiovascular disease at 6.7%. For clinical studies currently in progress, neuropsychiatric and chronic diseases accounted for the majority of indications; however, it has been shown that the scope of DTx is expanding with indications for neurological diseases as follows. Neurodegenerative diseases such as Parkinson’s disease, mastectomies, cancer diseases such as lump resections, blood diseases such as multiple myeloma, solitary plasmacytoma, plasma cell diseases such as amyloidosis, multiple sclerosis, multiple sclerosis-related depression and anxiety, fibromyalgia, back pain, chronic diseases such as heart failure (different from existing chronic diseases), autism spectrum disorders, schizophrenia, major depressive disorder, heart failure, pain, acute postoperative pain, systemic diseases such as lupus erythematosus, and dysarthria after stroke15,16.
For national clinical trial registration, the United States registered the most with 27 cases (60%), followed by Switzerland with two (4.4%), Finland with three (6.7%), South Korea with two (4.4%), Italy with two (4.4%), Brazil with one (2.2%), Malaysia with one (2.2%), Singapore with one (2.2%), Canada with one (2.2%), Israel with one case (2.2%), and Poland with one case (2.2%). three case (6.7%) had no clinical trial information on the ClinicalTrials.gov website. Clinical research was conducted by various institutions, including 24 companies (53.3%), four pharmaceutical companies (8.9%), seven universities (15.6%), four hospitals (8.9%), three research institutes (6.7%), two medical consortiums (4.4%), and one other (2.9%).
Figure 1 shows a Sankey diagram analyzing clinical studies registered on ClinicalTrials.gov or on DTA website, or clinical studies that are not registered on ClinicalTrials.gov but presented through research papers. This diagram shows the relationship between clinical trial registration or publication timing, indications, sponsor type, country, clinical trial type, study design method, and primary outcome. Figure 1 shows that the types of indications have been diversifying over the years, showing that companies are prioritizing clinical trials more proactively. In addition, more than half of clinical trials were conducted in the US. Most clinical trials were conducted with randomized controlled trials (RCTs). For clinical study design, parallel had the highest proportion. The primary outcome included all of the general medical practice areas such as treatment, diagnosis, and prevention, but the proportion of treatment was the highest, followed by research. The proportion of other primary outcomes was insignificant.
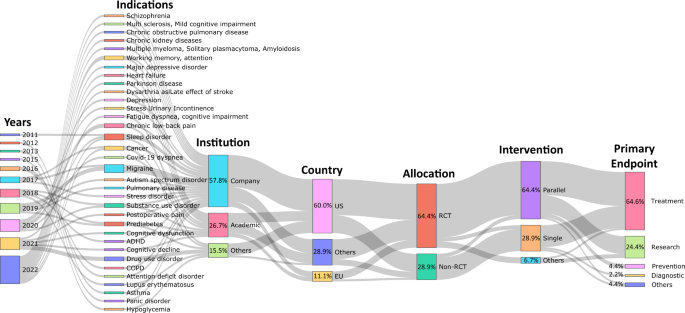
A Sankey diagram was utilized to analyze trends in indication, institution, country, trial type, intervention, and major outcomes. Between 2010 and 2022, a total of 31 clinical trials related to digital therapeutics were identified on the ClinicalTrials.gov website using the keyword “Digital therapeutics”. The clinical trials were conducted by a variety of institutions, including pharmaceutical companies, hospitals, research institutes, medical consortia, universities, and corporations. These trials were carried out in multiple countries, including the United States, Europe, Switzerland, Finland, South Korea, Italy, Brazil, Malaysia, Singapore, Canada, Israel, and Poland. The clinical trials employed interventions such as parallel, single, crossover, and factorial designs, with primary objectives that included treatment, research, prevention, diagnosis, supportive care, and basic science.
Commercialization of digital therapeutics
Many countries are promoting the commercialization of DTx through multi-sector cooperation with regulatory authorities, pharmaceutical companies, and medical experts. In the initial stage, DTx are approved and commercialized primarily for chronic diseases such as diabetes, cardiovascular disease, respiratory disease, and chronic pain or neuropsychiatric diseases such as drug addiction, sleep disorder, schizophrenia, chronic pain, and attention deficit hyperactivity disorder (ADHD)3. However, DTx for more diverse indications, such as irritable bowel syndrome17, migraine18, hip and knee replacement surgery, and ear disease, have been launched recently. Most DTx have been released through Food and Drug Administration (FDA) clearance led by the US (see Table 3), and currently, CE marked DTx products are being launched in Europe, primarily in Germany and Belgium1.
A representative example of commercialized DTx is reSET (Pear Therapeutics Inc., MA, USA). reSET is the first interactive FDA cleared DTx for cognitive-behavioral therapy of drug and alcohol addiction patients. reSET provides a professional online counseling service and a face-to-face treatment service with medical staff according to the results of a patient self-questionnaire. In addition, EndeavorRx (Akili Interactive Labs Inc., MA, USA), developed as a DTx for pediatric ADHD, demonstrated that it can improve a patient’s attention index (API) by stimulating and activating the prefrontal cortex through video games19, and FDA-510(k) clearance and CE mark were obtained. Teva Pharmaceuticals’ Propeller Health (ResMed (Propeller Health), WI, USA)20, and ProAir Digihaler (Teva Pharmaceuticals Inc., NJ, USA)21, which are medication reminders with an inhaler, have proven to have a 79% reduction in inhaler use when applied to asthma and COPD patients, respectively, and obtained FDA-510(k) certification22. In addition, Sleepio (Big Health, CA, USA), developed for the improvement of sleep disorders, showed that the treatment effect can be improved from 20 to 76% through the sleep management function and online sleep disorder counseling program23.
Unlike general medicines, DTx operate by providing treatment content through computer or mobile applications. Because implementation technologies such as web or mobile app development and server construction do not have much differentiation for each indication, the specific treatment effect is determined by the content and application method provided. Therefore, each company’s DTx pipeline will depend on how the company will provide indication-specific content to the DTx platform it has already secured. Development of DTx contents for each indication is primarily performed through the company’s own development or in cooperation with research institutes such as universities or hospitals, and DTx are expanded to various indications by continuing cooperation with existing partnerships or establishing new partnerships with specialized institutions.
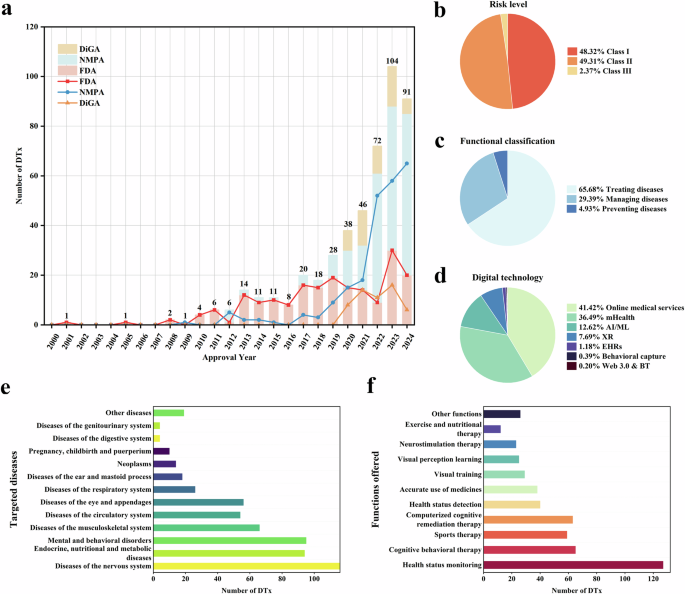
Figure 2 shows a Sankey diagram of commercialized DTx products, year of release, indication, regulatory authority, and regulatory class. The number of commercial products released has a repeating pattern of an increase and a decrease every two years since 2015, but shows an overall increase. Many of the products initially released were related to chronic or neurological diseases, and products released after 2020 were observed to be related to psychiatric disorders. By regulatory authority, most products are FDA cleared and CE marked. A total of 51 products received CE mark or FDA clearance, of which the number of CE marked products (26) was approximately double the number of FDA cleared products (14), and 14 products obtained both FDA clearances and CE marks. Most of the FDA cleared products obtained Class II grade certification, whereas most CE marked products were certified as Class I grade (Supplementary Table 2). However, it is unreasonable to generalize that CE legislation is stricter than FDA legislation; therefore, there are many approvals of low-grade medical devices. Recently, the EU has strengthened regulations from MDD to MDR, and one questionnaire to industry found that 89% of respondents now prefer US rather than EU market entry for innovative devices due to the increased predictability of regulatory requirements24. The European DTx approval flow is expected to change after 2021, as the difficulty of obtaining new product approvals has increased, and strict post-marketing surveillance is involved.
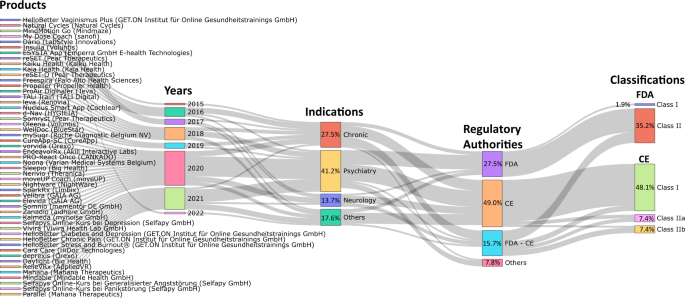
Sankey diagrams were employed to analyze trends in the types of indications, regulatory agency types, and class types for commercial digital therapeutic devices. The investigation focused on products listed on the DTA, DiGA, and mhealthbelgium websites, as well as products that had received FDA approval listed in the FDA medical device database. Regulatory authorities for each product were also investigated. Commercial digital therapeutics are primarily launched for indications related to chronic conditions, psychiatry, and neurology, with additional products available for urinary incontinence, ear disorders, hip and knee arthroplasty, tinnitus, irritable bowel syndrome, and vaginismus. Most commercial products have received approval from the FDA and CE regulatory agencies, while others have been approved by Japan’s Ministry of Health, Labour and Welfare (MHLW) and the Medicines and Healthcare products Regulatory Agency (MHRA).
Regulation of digital therapeutics
Six guidelines and one policy document were investigated for DTx-related regulations within the International Medical Device Regulators Forum (IMDRF) member jurisdictions. The FDA has stated that it will permit the distribution and use of devices during public health emergencies without filing a premarket notice under section 510(k), as the FDA considers that DTx do not pose undue risk25. Six guidelines were published by the Ministry of Food and Drug Safety (MFDS, South Korea). The guidelines published in 2020 provided guidance on the definition of DTx and documents required to be submitted when obtaining DTx approval in the Korean regulatory system26. Subsequently, five guidelines published from 2021 to 2022 suggest safety and performance evaluation methods for DTx for alcoholism27, depressive disorder28, insomnia29, nicotine use disorder30, and panic disorder31 and provide design examples of clinical trial protocols with primary endpoints, sample size, and hypothesis for each guideline. Although there are no specific guidelines, except in Korea, the continued cases of approval through regulatory authorities in the United States, Europe, and Japan suggest that approval is possible within the current regulatory system for general medical devices. However, researchers are suggesting the establishment of a regulatory system suitable for DTx. Researchers argue that the regulatory system of DTx is considerably vague32 or insufficient33, and Vilardaga et al. indicated the limitation that DTx regulation does not guarantee usability and continuous adoption14. A stakeholder study on the development of the DTx industry reported that it is important to develop guidelines for permits and prepare a simplified regulatory system following research funding, suggesting that the role of the government is important34. In addition, researchers urged the development of an internationally harmonized regulatory model to improve the safety and quality of DTx35,36,37.
link