Prospective validation of a mobile health application for blood pressure management in patients with hypertensive disorders of pregnancy: study protocol for a randomized controlled trial | Trials

Explanation for the choice of comparators 6b
The participants follow the 2022 Korean Hypertension Treatment Guidelines [14]. These guidelines recommend ambulatory blood pressure monitoring for pregnant individuals with hypertension to avoid unnecessary medication. Pharmacological treatment is initiated for severe hypertension (≥ 160/110 mmHg), aiming to lower blood pressure below 150/100 mmHg without reducing diastolic pressure below 80 mmHg excessively. Postpartum, efforts are made to maintain blood pressure below 140/90 mmHg. The choice of medication considers prior usage, side effects, and fetal risk.
Intervention description 11a
All study participants receive the standard care and continue to receive the same proactive treatment. They are provided with OMRON automatic blood pressure monitors and are instructed by the research nurse to measure their blood pressure once daily in a quiet environment; after a minimum of 5 min of rest, participants will undergo three consecutive blood pressure measurements, each taken at 5-min intervals. The mean of the second and third measurements is recorded. Based on the measured blood pressure, if necessary and at the discretion of the treating physician, adjustments or new prescriptions for antihypertensive medications will be made.
In the intervention group, participants also receive education from the research nurse on installing and navigating the Heart4U application. At the registration point and every 2 weeks thereafter, the usage and input levels of all participants will be monitored. Participants who log in less than three times in a 2-week period will be contacted to address any issues they might face with application usage. Furthermore, in the group using the Heart4U application, medical professionals will review the information recorded in the app through the integrated electronic medical records during antenatal visits. Both groups undergo observations in accordance with the antenatal examination schedule, and the clinical follow-up period will be 1 month after childbirth.
Criteria for discontinuing or modifying allocated interventions 11b
Participants have the autonomy to discontinue their involvement in the trial at any juncture without needing to provide further explanations. In the event of participant withdrawal, missing data will be handled through inverse probability weighting and worst-case imputation schemes as part of the sensitivity analyses. Additionally, no crossover from the control arm to the intervention arm will be permitted during the follow-up period. Furthermore, even in the event of crossover from the control arm to the intervention arm, follow-up will continue as per the intention-to-treat (ITT) analysis protocol.
Strategies to improve adherence to interventions 11c
Adherence in this study is determined by the frequency of blood pressure measurements and the regularity of logging into the Heart4U application in the intervention group. The study team monitors adherence during clinical visits scheduled for antenatal examinations. To enhance adherence, the research nurse conducts regular assessments during clinical visits, ensuring that participants encounter no difficulties with using the blood pressure monitor and the application. Additional education is provided as needed to address any challenges and optimize participant engagement with the intervention.
Relevant concomitant care permitted or prohibited during the trial 11d
No relevant concomitant care is specifically permitted or prohibited. Managing blood pressure in pregnant individuals can be challenging due to the potential impact on utero-placental circulation and the risk of fetal anomalies associated with various medications. Given these considerations, there are typically no additional restrictions imposed on concomitant care in this trial to ensure that participants receive comprehensive and appropriate management for their HDP.
Provisions for post-trial care 30
No provisions are anticipated for post-trial care.
Outcomes 12
Primary outcome
The primary endpoint of this study is the difference in SBP measured between the point of enrollment and the conclusion of the study, which is 1 month ± 1 week after childbirth.
Secondary outcomes
-
(1)
Trajectory blood pressure: Throughout the study duration, trajectories of systolic, diastolic, and mean blood pressure will be tracked.
-
(2)
Obstetric outcomes: This includes but is not limited to:
-
Antihypertensive medication use
-
Progression to severe preeclampsia or eclampsia
-
Pulmonary edema
-
Fetal growth restriction
-
Oligohydramnios
-
Preterm placental abruption
-
Preterm delivery (between 20 and 37 weeks)
-
Miscarriage (before 20 weeks)
-
Fetal demise
-
Newborn weight
-
Low birth weight
-
Postpartum hemorrhage (including transfusion)
-
-
(3)
Trajectory of body mass index (BMI): analysis of BMI trajectory.
-
(4)
Physical activity monitoring: step counts recorded through Samsung Health or iPhone-Health applications.
-
(5)
Depressive Mood Assessment: depressive symptoms will be assessed using surveys, including the Patient Health Questionnaires-9 and Beck Depression Inventory [15, 16], administered at enrollment and at the conclusion of the study. These tools are widely recognized and validated measures for evaluating depression symptoms and severity.
-
(6)
Drug adherence: evaluation of medication adherence through pill counts
Rationale for outcomes
Our research aims to provide active support for enhanced blood pressure control in the context of HDP, thereby improving outcomes associated with this condition. By aligning the convenience of technology with the challenge of managing HDP, this study endeavors to positively affect the health and well-being of both mothers and infants. Through comprehensive monitoring and assessment of various outcomes, including blood pressure trajectories, obstetric outcomes, physical activity levels, mood, and medication adherence, we seek to gain insights into the effectiveness of our intervention in mitigating the adverse effects of HDP and promoting overall maternal and fetal health.
Participant timeline 13
The participant timeline is shown in Fig. 3.
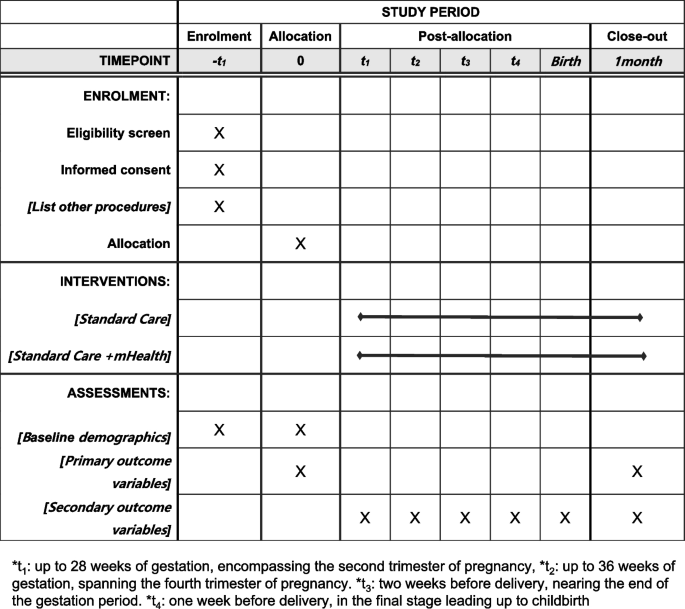
Timeline for participants
Sample size 14
The determination of the sample size for this study, with an intended enrollment of 580 participants, was established using specific parameters as follows [17, 18]: the mean SBP at the point of enrollment was 146 mmHg, accompanied by a standard deviation of 13 mmHg. Subsequently, the mean SBP 1 month after childbirth was projected to be 130 mmHg, with a corresponding standard deviation of 12 mmHg. The control group displayed a delta SBP of 16 mmHg, whereas the delta SBP of the intervention group was amplified by 20% to 19.2 mmHg. The required difference between the two groups for sample size computation was 3.2. Moreover, a standard deviation of 13 mmHg was attributed to the delta SBP. These considerations are further coupled with an alpha error of 0.05 and a power of 80%. Using these variables, the calculations stipulated the necessity of 261 participants within each group, culminating in a total of 522. Considering a presumed dropout rate of 10%, the required comprehensive sample size was 580 participants.
Recruitment 15
Recruitment for our trial targets pregnant individuals diagnosed with HDP who are attending our hospital’s high-risk maternal care center. Screening for eligibility criteria is conducted by the attending obstetrician, who verifies whether the patients meet the specified criteria. Patients receive a comprehensive explanation of the trial’s risks and benefits to ensure their full understanding before being asked to provide voluntary written consent for participation. This process aims to ensure that participants are fully informed and willing to enroll in the study.
link