Approved trends and product characteristics of digital therapeutics in four countries
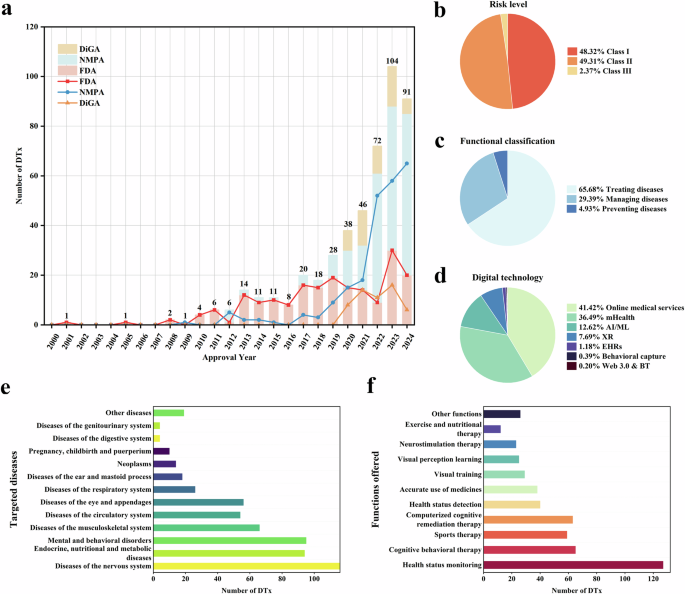
This study reveals significant geographical heterogeneity in the regulatory patterns and market development of DTx across four countries. Prior to 2020, the approval of DTx products worldwide was mainly concentrated within the FDA (Fig. 1a). The US has been promoting the concept of DTx since 201227, launching a pre-certification program for digital health in 201728,29 and providing a fast track for the approval of DTx products. In the same year, the FDA approved Pear Therapeutics’ reSET through the “Innovative Medical Devices (De Novo)” pathway. This was the first DTx product targeting drug abuse and alcohol dependence. These policies might have led to a sharp increase in the number of FDA-approved DTx in the US. However, a decline had also emerged in recent years, with only 20 DTx approved in 2024, reflecting the challenges faced by the industry. Challenges might include difficulties with clinical validation and marketing operations, the exploration of business models, and issues with the stability of the funding chain30,31. Three prominent DTx companies that went public during the rapid expansion of the industry from 2021–2022 have exited cthe apital markets consecutively. Pear Therapeutics filed for bankruptcy in April 202332 and Better Therapeutics and Akili Interactive filed for delisting in March and May 2024, respectively33,34.
Germany and Belgium have actively promoted the approval and application of DTx. Germany implemented the Digital Healthcare Insurance Law in December 2019. This law was the legal foundation for DTx approval and, for the first time, incorporated prescription apps into the healthcare insurance system. Belgium launched the mHealth Belgium platform in 2018, establishing a hierarchical approval framework for medical applications and providing reimbursements to promote the application of DTx. However, DTx approvals in Germany and Belgium have slowed significantly in the past year, which may reflect the standardization and strictness of the approval process.
Compared with these mature international systems, the regulation of DTx in China is in its developmental stage. China has not yet formulated regulatory documents specifically applicable to DTx but has instead used the existing approval system for medical device software. Although China entered the DTx field relatively late, the number of approvals has been increasing yearly, which may be related to government-led initiatives to accelerate DTx development. For example, in 2021, Hunan Province took the lead in significantly reducing the time from application to the final approval of Class II medical devices (from 80 to 40 working days)35, prompting most DTx companies to develop in the Hunan Province (corresponding to the darkest area in Fig. 3b, n = 72). In the same year, Zhejiang Province established the first domestic DTx industrial park, and Hainan Province included exploration of DTx in its provincial development plan for 2022.
The market performance of DTx is influenced by various factors, including clinical trial risks, product acceptance, insufficient academic research, and limited confidence among physicians. Existing evidence indicates that the decline in DTx approval numbers in the US, Germany, and Belgium may be linked to these factors13,36,37,38. In contrast, China has caught up through a government-led model, with its annual approval volume surpassing that of the US after 2020, reaching 65 in 2024. However, the long-term impact of China’s government-led model remains unknown. To avoid similar challenges encountered by other countries in DTx promotion, China needs to incorporate evidence-strengthening mechanisms into its policy design. Currently, China has planned to enhance its policy in this regard, including incorporating DTx into the medical service project catalog, encouraging hospital cooperation pilots, and strengthening clinical validation requirements39. These measures are expected to promote business model implementation and improve patient and physician acceptance, but their long-term impact still requires systematic evaluation.
Regarding indications, DTx related to cognitive impairment and ophthalmology dominates the Chinese market, whereas the US, Germany, and Belgium value products for chronic disease management and mental disorders (Fig. 4). The development of DTx for cognitive impairment in China may be due to rapid population aging, which has resulted in an overall increase in the number of patients with cognitive impairment. In 2023, the population aged ≥65 years in China comprised 217.09 million people40. The development of DTx in ophthalmology has primarily focused on the prevention and control of amblyopia in children and myopia in adolescents. Owing to the treatment cycles for these conditions, prevention and control are characterized by early treatment and recovery, directly affecting parents’ willingness to pay. Considering dietary and lifestyle habits, the US, Germany, and Belgium have larger populations of patients with chronic diseases. Studies have shown that 86% of medical resources in the US are invested in chronic disease management41, resulting in a significant market share of DTx products related to chronic disease management. Additionally, we found that China, the US, and Germany have a significant number of DTx treatments targeting mental and behavioral disorders (China: n = 48, 20.43%; the US: n = 20, 10.42%; Germany: n = 26, 47.27%). However, China tends to focus more on sleep disorders, whereas other countries pay more attention to mental disorders, such as depression and anxiety.
DTx has gained widespread acceptance from physicians’ professional associations; however, these associations emphasize the need for robust clinical evidence and data security. For example, the American Medical Association (AMA) is supportive of DTx but stresses the need for more clinical research and data to validate its safety and efficacy42. In Europe, the medical community has shown strong interest in DTx, although clinical applications require further validation43. In the UK, the National Health Service (NHS) has been proactive in promoting DTx, particularly for mental health and chronic disease management44. In China, with the rapid development of digital health technologies, the healthcare industry’s awareness and acceptance of DTx is gradually increasing, and DTx applications are becoming more widespread12. Better clinical effectiveness demonstrated through real-world evidence, coupled with clinician confidence, will be the primary reason for adopting DTx. Payment incentives and other policies will be major drivers for their implementation. Although overall satisfaction still requires improvement, acceptance among patients is gradually increasing as clinical evidence and usage outcomes accumulate45,46. For example, in the field of mental health, DTx has effectively reduced patients’ stigma caused by social prejudice through anonymization and virtualization, significantly improving treatment adherence and satisfaction among patients with mental illness47. Regarding chronic diseases, the collateral advantages of DTx compared with those of traditional medications are significant. Software-driven interventions based on behavioral changes and lifestyle adjustments reduce the possible toxic side effects of traditional medications, thereby improving medication adherence48. In addition, DTx—with the help of remote-monitoring technology—can track patients’ health data in real time and provide them with personalized health management plans, effectively improving their health outcomes49.
Economic factors play a significant role in the adoption and implementation of DTx across different healthcare systems. The expansion of health insurance coverage, flexible reimbursement policies, value-based payment models, and employers’ attention to employee health provide strong support for the promotion of DTx. For example, health insurance reimbursement policies in Germany and France50 and the application of current procedural technology (CPT) codes in the US42 enable physicians and employers to receive economic compensation for the use of DTx, thereby increasing the adoption rate. Conversely, these factors may hinder the adoption of DTx. For instance, reimbursement restrictions on software products by Medicare in the US and the lack of widespread coverage of DTx products by public insurance in South Korea and Japan limit market promotions20,51. Additionally, the effectiveness of employer-sponsored programs is constrained by unclear cost-effectiveness and low employee participation.
As an emerging patient-centered medical approach that combines the latest digital technologies, DTx has enormous development prospects. However, many challenges remain (e.g., the difficulty of achieving scale, the low degree of marketization, and insufficient recognition from doctors and patients). The challenges are related to the characteristics of DTx52. First, the development of DTx is difficult, and efficacy requires extensive clinical research and validation, as well as close involvement and continuous research from the academic community. Our PubMed search produced approximately 1000 results. Quite a few were not research articles, indirectly showing that DTx are significantly under-researched compared to their potentially huge impact on patient care. Although the study findings indicate that regulatory agencies and manufacturers attach considerable importance to DTx, the relative lack of attention from academia and clinicians has influenced the lack of widespread acceptance. Simultaneously, in the research and development process, privacy protection and security of patient data must be core considerations53. All these factors incur high costs for manufacturers. The complexity of payment mechanisms also leads to ambiguity among payers. Second, DTx takes a long time to work, with no breakthrough change in the therapeutic effect. Unlike drugs, DTx works by changing patients’ behaviors and lifestyle habits over the long term, requiring more substantial cooperative efforts54,55,56. Furthermore, DTx takes up doctors’ time and poses joint liability problems. Doctors must invest time and effort in intervention and guidance (creating work pressure for doctors who have a joint effect on DTx prescriptions). Currently, their willingness to prescribe DTx is not high57. Finally, the boundary between DTx and other digital health products remains unclear, and the complexity of the technical review process poses significant challenges for approval and regulation58.
Resolving these issues requires joint efforts from all parties involved. Manufacturers should continue to promote technological innovation, explore diverse business models59, and strengthen market promotion activities to enhance doctors’ awareness and acceptance of DTx. Relevant approval and regulatory systems should be improved to accelerate approval and effective supervision. Drawing on Germany’s DiGA fast approval process, regulators can implement reasonable clinical trial exemption policies and establish a dedicated DTx database to improve approval efficiency. In addition, the policy support system for DTx should be improved, the definition and standards of DTx should be clarified, and a sound payment system should be established to provide clear guidance for market access to and reimbursement of DTx20. From the user’s perspective, the most significant deficiency is the insufficient awareness and understanding of DTx efficacy among clinicians and patients. Currently, market and regulatory authorities attach importance to DTx while users are passive. Therefore, the academic community must increase relevant research, promote breakthrough innovations in DTx technology, increase evidence of efficacy, and ultimately improve awareness and acceptance among clinical doctors and patients.
This study has some limitations. First, owing to the lack of uniformity in the definition of DTx among countries and inconsistent data formats and levels of openness, the data collection and integration process was complex. Although efforts were made to ensure the completeness and accuracy of the data, some deviations may still exist (e.g., the lack of accurate approval time for DTx on the platform for Belgium may lead to integration bias in the dataset). Second, although the descriptive statistical analysis method used in this study provided a basic understanding of the approval status of DTx in the selected countries, it may not be sufficient to fully reflect the overall development trend of DTx worldwide. Future research should explore how to address the bottleneck issues associated with DTx, including how to promote behavioral change, gain support from doctors, improve patient compliance, and maximize treatment outcomes, to explore mature, sustainable development models. Similarly, future research could delve into other countries with distinctive healthcare systems (e.g., the United Kingdom, France, emerging countries in Africa, Asian economic powerhouses such as Japan and Korea, and countries with complex geographical conditions, such as Russia and Canada). Analyzing the current state of DTx development in these countries, identifying their unique influencing factors, and integrating these data into our database can provide the industry with a more comprehensive reference, thereby more effectively advancing the development of the DTx sector.
link







