Attitudes of healthcare professionals and researchers toward wearable and app derived patient generated health data
Search results
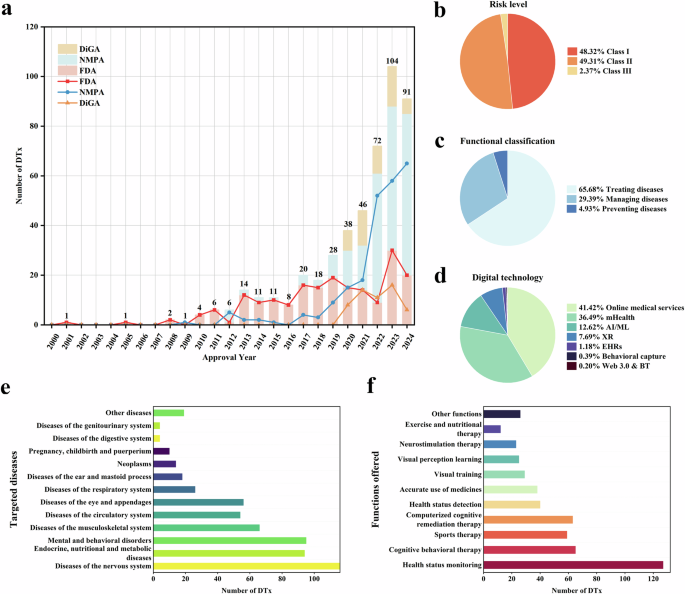
A total of 299 records were retrieved from the electronic databases PubMed, Embase, and Google Scholar (Table 1). After removing duplicates, 246 articles remained for the title-abstract screening, of which 25 were included for the full-text screening. Of these, 15 met the inclusion criteria8,9,10,11,12,13,14,15,16,17,18,19,20,21,22. Six additional articles were identified through a hand search on Google23,24,25,26,27,28 and 12 through reference tracking of included studies (forward tracking29,30,31,32 and backward tracking33,34,35,36,37,38,39,40). A total of 33 studies were included in the thematic analysis8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40. Title–abstract screening and full-text screening reasons for exclusion are summarised in Supplementary Information Table 2.
Characteristics of included studies and quality assessment
Table 2 summarises the key characteristics of the included studies. The complete data extraction including recruitment information, non-healthcare professional participant groups, data collection and analysis can be found in Supplementary Information Table 3. Of the 33 studies, 24 had a qualitative8,9,10,11,14,16,17,18,19,20,21,22,24,26,27,28,30,31,33,35,36,38,39,40, six a quantitative12,13,15,25,29,34 and three studies had a mixed methods study design23,32,37. Most qualitative studies (19/24) were based on interviews9,10,11,14,16,18,19,20,21,22,24,26,27,28,31,33,38,39,40, and the rest (5/24) used focus groups17,35, group discussions8,30 or a card-sorting session36. Mixed methods studies combined surveys with interviews (2/3)23,37 or focus groups (1/3)32. All quantitative studies were based on surveys for data collection12,13,15,25,29,34.
Most studies (22/33) recruited a mix of healthcare professionals for their data collection, including physicians, psychologists, therapists, and nurses among others8,9,10,11,13,16,17,18,20,21,22,25,28,31,32,33,34,38,39. Eight studies focused on physicians only12,15,19,24,26,30,37,40. Three studies specifically focused on the perspectives of researchers and will be separately analysed and can be found in the Supplementary Information Note 314,23,27.
Fifteen studies explored opinions toward health apps and/or wearables generally8,15,16,19,22,23,24,26,27,28,30,31,33,39,40, and 18 focused on a specific disease indication9,10,11,12,13,14,17,18,20,21,25,29,32,34,35,36,37,38, such as mental health conditions21,36 or cancer10,25.
Six studies9,11,18,28,37,38 investigated perspectives of HCPs within a clinical trial/research programme piloting apps and wearables for PGHD collection for patient care (Table 3). One study used a medical device app to monitor ECG in patients with Atrial Fibrillation38. Another study investigated a prototype medical device to monitor diabetes and two studies explored non-medical device data handling apps to track symptoms of rheumatoid arthritis based on validated questionnaires9 and an image-based wound healing tracker for post-discharge surgical site infection18. HCPs in those studies accessed the shared PGHD through web dashboards/portals18,38 or reports within9 or separate from the EHR37. Another study by the U.S. Department of Veterans Affairs piloted the fitness and activity tracker Fitbits for veterans that synced the PGHD via a data sync app to a web-based provider dashboard28. Another study interviewed HCPs from five different studies with focus on PGHD sharing. In these studies, PGHD were collected using various, unspecified consumer mHealth devices, with provider access either facilitated through a special dashboard/platform or at the patient’s discretion, e.g., during consultation on their device11. In 24 studies, the opinions on PGHD were assessed without actually sharing PGHD; of those, 23 did not differentiate the type of device for data collection.
Table 4 summarises the detailed quality assessment of all included studies. All studies passed the initial screening questions “Are there clear research questions?” and “Do the collected data allow to address the research question?”. The methodological quality of the included studies was medium. The most frequently found issue in studies with quantitative methods was the risk of sampling bias and non-response bias12,13,15,23,25,29,32,34,37. The most frequent issue with qualitative methods in studies was the underreporting of quotes to prove findings. Two studies stood out because of their very low number of HCP participants of five8,16,17,23,27,30 or two9, respectively.
Thematic synthesis
The thematic synthesis yielded a multitude of analytical themes that were grouped into five main categories: 1) Benefits of PGHD for patient care; 2) Improving patient management and clinical workflows; 3) Barriers to use PGHD; 4) Evolving roles of patients and HCPs in a changing healthcare system; and 5) Researchers perspectives on PGHD in medical research. The following sections describe the results for categories one to four. The fifth category is separately analysed and can be found in Supplementary Information Note 3.
Benefits of PGHD for patient care
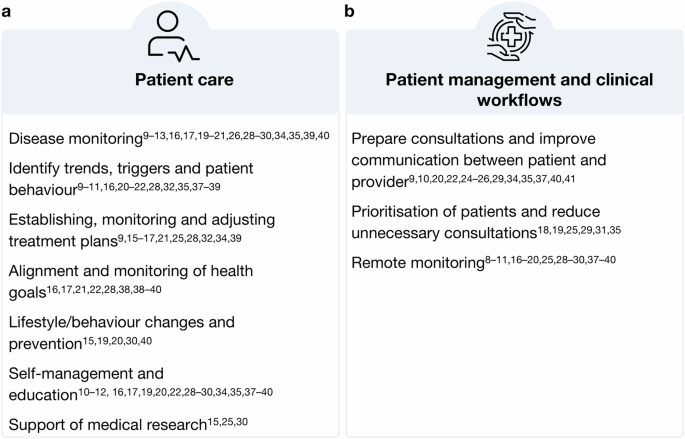
HCPs in various medical specialities and professional groups across studies identified various benefits that access to outside-of-clinic PGHD from health apps and wearables could provide by filling long-existing gaps in the traditional clinical data. Figure 1a summarises the findings. Insights derived from PGHD can support the monitoring and understanding of disease progression and the overall health status (1)9,10,11,12,13,16,17,19,20,21,26,28,29,30,34,35,39,40. Continuous data collection can help to identify trends, triggers and behaviours impacting patient, health (2)9,10,11,16,20,21,22,28,32,35,37,38,39 Further, PGHD can be used to establish, monitor and adjust treatment plans (3)9,15,16,17,21,25,28,32,34,39, enabling collaboration between patient and provider to align and monitor the health goals of patients (4)16,17,21,22,28,38,39,40. PGHD utilisation is further seen as valuable for supporting lifestyle and behaviour changes and promoting prevention (5)15,19,20,30,40 and empowering patients to self-manage their diseases (6)10,11,12,16,17,19,22,28,29,30,34,35,37,38,39,40. In addition to its potential in patient care, HCPs in three studies also highlighted PGHD value for medical research (7)15,25,30. Two of those studies were quantitative: one with cancer care physicians found that 93% of participants supported using oncology app data for research25, and another with physicians from 36 different medical specialities found that 56% saw benefits or medical research15. Overall, HCPs found that PGHD utilisation offers significant benefits for both patient care and medical research by providing continuous, personalised health insights and fostering a collaborative approach to health management.
a displays benefits in patient care and (b) in patient management and clinical workflows. (Author’s summary).
Four studies quantitatively assessed which types of PGHD HCPs considered as useful. For this review, PGHD types rated as useful by at least 50% of the surveyed HCPs qualified for the reporting. For epilepsy patients, heart rate, sleep quality, body movement29,34, breathing rate, mood and concentration29 were identified as useful. Sleep quality was also considered valuable for mental health and multiple sclerosis patients29. In geriatric care, HCPs rated heart rate, blood pressure, blood glucose, weight, body fat, body/skin temperature, physical activity, sedentariness, step count and electrodermal activity as useful32. In a study involving oncology care physicians, useful features for an oncology app would allow the tracking of side effects, quality of life, test results, and treatment satisfaction25. A few studies report on patient-reported outcome measures (PROMs), a subset of PGHD that is collected through standardised and validated questionnaires10,11,21,24,25,32,33. One study with mental healthcare professionals highlighted the value of combining PGHD from different sources, e.g., PROMs and app behavioural data, for a holistic view of a patient’s health status21. In contrast, GPs in another study valued PROMs collected through their own clinical system more valuable than other types of patient-collected data, such as wearable data24. For oncology patients, app-collected PROMs such as quality of life25 and therapy-related measures10 were considered valuable. One study specifically excluded PROMs from their investigation11. Table 3 shows a summary of all devices and respective PGHD types shared and/or discussed in the studies.
Despite the anticipated benefits of PGHD in patient care, HCP participants across the studies expressed mixed opinions about its nature, including both actively- (user input) and passively-sensed data. GPs of one study equated heart and sleep data from wearables to verbal symptom descriptions, not as concrete measures24. Conversely, others viewed PGHD as a more objective and potentially accurate source of medical information20,26,35, comparing it to “hard data”28 and considering it less prone to recall bias35,37,39. This digital data can also help avoid “doctor-pleasing” bias, where patients report what they think physicians want to hear16,37. Some HCPs appreciated the subjective nature of PGHD as a feature, acknowledging that it requires an understanding of how individual patients express themselves39.
For instance, a cardiologist in the same study described that managing atrial fibrillation often relies on patients’ subjective symptom experiences39. Additionally, a HCP in another study found that PGHD can provide a holistic view of a patient’s daily life, offering insights beyond what can be discussed in a single clinical visit and fostering greater empathy with patients11.
Even when PGHD did not directly impact care plans37 or was not seen as valuable evidence24,35, HCPs still found it a useful starting point for patient conversations and setting the consultation agenda24,35,37. The initiation of the PGHD tracking itself can help HCPs understand intrinsic patient behaviour and motivation22,39.
Improving patient management and clinical workflows
In addition to its various benefits for patient care, HCPs across studies highlighted the potential of PGHD to enhance patient management and streamline clinical workflows. Figure 1b provides a summary of these findings.
In several studies, HCPs noted the utility of PGHD in preparing consultations. By tailoring appointments to specific patient issues identified in the PGHD, consultations could become more patient-centred and efficient9,10,20,22,25,29,37,40. For example, 77.8% of oncology care professionals in one study anticipated more efficient consultations with PGHD access25, while only 18% of HIV-care professionals in another study expected similar time savings12. In a study piloting a diabetes report, HCPs described a possible successful PGHD integration in clinical workflow by standardising and automating patient self-assessment and providing HCPs with a structured report, preferred directly in the EHR system before the consultation37. A study involving rheumatoid arthritis patients who tracked daily, weekly, and monthly symptoms in an app directly synced with the EHR found that accessing this data during consultations was considered useful by treating HCPs9. The longitudinal view of the patient’s health status provided by the app was seen as a potential time-saving alternative to standard disease history-taking during consultations.
HCPs in several studies identified the value of accessing PGHD between clinical visits. This data can help prioritise patients for follow-up visits and reduce unnecessary consultations18,19,25,29,31,35, freeing up crucial resources in an overburdened healthcare system. For example, HCPs considered post-discharge wound data tracked by at-risk-patients at home with their phone valuable for triage18. Additionally, PGHD can facilitate remote monitoring8,9,10,11,16,17,18,19,20,25,28,29,30,37,38,39,40, which is particularly useful for conditions like diabetes, where therapy adjustments can be made without in-person visits19,30. This approach helps manage increasing service demands and staff shortages effectively. Similarly to the diabetes case, a study on epilepsy, depression, and multiple sclerosis found that using patients’ devices to collect data at home and directly send it to the EHR system allowed for review between consultations if the system flagged reasons for concern29.
An important discussion point for many participants was the timing (when) and tool (how) for accessing PGHD. Many HCPs preferred access to the PGHD directly before a consultation20,34,37, while others favoured during the visit19,21,26,34,35 or in between appointments11,16,19,25,26,28. Regardless of timing, most HCPs who were open to using PGHD preferred it integrated into the electronic health records (EHR) to streamline their workflow8,9,10,11,15,16,17,18,19,20,21,22,25,28,33,37,39,40. Some suggested labelling PGHD separately from clinical data within EHRs28,33, though a minority preferred keeping raw PGHD out of the EHR28. Additionally, some HCPs were positive about accessing PGHD through patient portals16,22,33,34,38 or on the device during consultations19,21,22,25,26,34,39.
PGHD are often unfamiliar data types and structures for the HCPs, as they are collected with consumer health technologies that are designed for the general public, not a medical audience. Following from this, HCPs expressed the need to rearrange the data and allow tailored visualisation options to make the PGHD more comprehensive and actionable11,16,18,21,22,26,39.
Preferences ranged from summary reports with labels for highlights or out-of-range values18,26,37,40 to full data access28.
For remote access to PGHD, HCPs emphasised the need for clear protocols and responsibilities for dealing with incoming data and suggested building on existing workflow strategies18,22,25,34,37,40. Many called for dedicated nurses or care coordinators to pre-process PGHD before involving physicians8,17,18,22,25,29,34,37,40. Gerontologic care clinicians expressed a desire for decision support systems to pre-process data and issue alerts32. However, physicians in a study piloting a diabetes PGHD report preferred to receive the report directly37.
It is important to note that the report in this study was tailored to the specific information needs of the treating physicians. When oncology HCPs were surveyed about an alert feature in an app, 64.8% supported notifications for critical data entries25. Of those, 49% wanted an alarm for the treating physician within 24 to 48 hours, 40% preferred immediate alarms for the physician on duty, and 14% wanted an independent query system. Epilepsy care professionals were less supportive of real-time alarm systems, preferring to use PGHD for assessing seizure events before or during consultations rather than for real-time monitoring34.
Barriers to using PGHD in clinical practice
HCPs from various professional roles and medical specialities across studies, were enthusiastic about using PGHD to deliver better patient care, optimise patient management and streamline clinical workflows. However, the HCPs also reflected a variety of barriers and concerns when using this data. This shows a dual attitude of HCPs towards PGHD from health apps and wearables. Figure 2 summarises the barriers and concerns HCPs expressed in the studies included in this review.
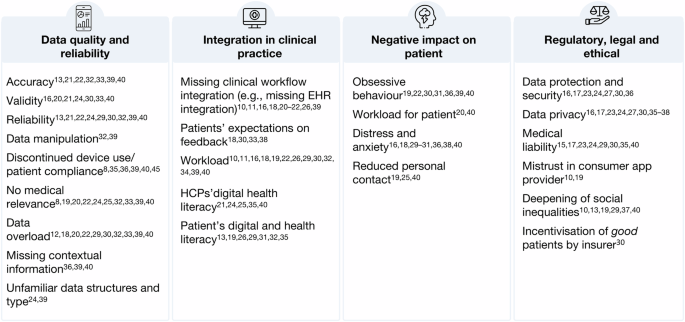
HCP concerns on integrating PGHD into care. Synthesised summary of HCPs’ concerns regarding the use of PGHD in clinical practice. (Author’s summary).
A central barrier to HCPs was the quality and reliability of PGHD and the impact on data evaluation. Across studies, worries around data accuracy13,21,22,32,33,39,40, validity16,20,21,24,30,33,40, and reliability13,21,22,24,29,30,32,39,40 were found as a major concern, indicating an underlying mistrust in consumer technologies. On one hand, this is rooted in HCPs not knowing how the devices work and how they measure data. On the other hand, HCPs doubt patients’ ability to use the device correctly and trustworthy. Some HCPs feared that their patients could purposely try to manipulate the data to mislead physicians and force specific actions, e.g. a specific diagnosis or insurance premium32,39. Additionally, just like in classic therapies, digital approaches are facing big challenges in patient compliance. A non-compliant or discontinued use of the device could make the PGHD further unreliable, as some HCPs noted13,29,32,33,38,40. While access to more data is generally valued, HCPs in many studies are worried about data overload12,18,20,22,29,30,32,33,39,40. This could become particularly stressful for HCPs when patients gather data deemed to have no medical relevance8,19,20,22,24,25,32,33,39,40. Regarding passively sensed PGHD, HCPs expressed concerns about the missing contextual information, which makes it difficult to interpret the data36,39,40.
Another big concern theme was the integration of PGHD into current clinical practice. In several studies, HCPs noted that PGHD are not integrated into the current clinical workflow, e.g., missing interoperability with running information management systems in clinics10,11,16,18,26,39. It is time-consuming and burdensome for HCPs to assess PGHD in a separate software from their EHR system. Another barrier is the workload10,11,16,18,19,22,26,30,32,34,39,40 caused by the additional data. Processing and reviewing the data would require additional time and staff resources, especially in remote monitoring settings. Asynchronously sharing PGHD with HCPs brings the additional challenge of patients’ expectations on response time18,30,33,38 and HCPs noted the need for a clear alignment (and control over) when and how patients would receive a response to shared PGHD11,18,38.
Another important concern raised by HCPs in several studies that made the integration of PGHD in clinical routines difficult was the varying levels of digital health and data literacy among healthcare professionals, which can negatively impact how they interact with the tools and the data collected by patients21,24,25,35,40. On the other hand, patients lacking digital health literacy were also of concern, as this can result in wrong application usage or misinterpretation of data13,19,26,31,32,34,35,40.
While apps and wearables are often celebrated as empowering tools for patients, HCPs in several studies worried about their potential negative impact on patient health. HCP worried that PGHD tracking could lead to obsessive behaviour in some patients or exacerbate existing tendencies in others19,22,30,31,36,39,40. The tracking could become an additional work for patients who already deal with a severe condition20,40. Here, HCPs not only recognise their own extra work but also respect the workload on the patient site. A constant engagement with one’s own health status can also cause distress and anxiety16,18,29,30,31,36,38,40, for example, when data shows unfavourable behaviour of the patient or certain goals are not achieved. The increasing digitalisation of the healthcare and the patient-provider relationship was also negatively seen by some HCPs who worried about reduced personal contact of patients with their HCPs could have on them19,25,40.
Another set of concerns can be grouped as regulatory, legal and ethical concerns connected to using PGHD. An often cited concern by HCPs related to data privacy11,12,18,19,22,25,30,31,34,40, protection and security11,12,18,19,22,25,29 issues, such as third-party access and storage of PGHD from consumer mHealth devices. Some HCPs were generally worried by the idea of using tools from commercial companies, so the source of the app or device mattered to them10,19. HCPs in several studies were further concerned about medical liability, especially about their responsibility of reviewing large amounts of data or using the information for patient prioritisation10,12,18,19,24,25,33,40. Another important concern raised by HCPs in several studies is the fear of deepening social inequalities among patients with varying socio-economic levels10,13,19,34,37,40. Patients of lower socioeconomic status often have limited access to technology, including the internet and devices, and can lack the ability to use digital tools effectively. As a result, patients who would benefit the most can not participate or can participate less effectively. This could be further exacerbated in a scenario identified by HCPs in a Swiss study30. They worried that apps and wearables could be used by insurers to incentivise and reward “good patients” for achieving health goals while leaving other patients with higher needs for activation behind.
HCPs recommendation to address PGHD challenges
To address concerns around PGHD quality and reliability, HCPs in several studies demanded evidence proof for apps and wearables to be used in clinical care8,10,15,26,33. Making this information available to PGHDs would help them to identify appropriate tools and boost trust to use them with their patients. Greater involvement of HCPs in developing apps and devices could further enhance clinical usefulness13,30 and allow for the integration of customised features like tailored tracking features or data displays11,18,26,28,39.
To integrate PGHD in clinical workflows, the majority of HCPs wished to access the PGHD directly through their EHR system or patient portal15,17,18,20,22,25,28,32,33,34,37. HCPs in one study, however, expressed the need to clearly label the source of this information to differentiate it from other forms of clinical data33. For remote monitoring settings, HCPs suggested a dedicated nurse or care coordinator who could preprocess data and alarm physicians accordingly8,17,18,22,29,34. This suggestion builds on existing clinical structures where nurses are often the first point of contact for the patients. Clear reimbursement plans were also recommended by HCPs in several to compensate the extra work created by guiding patients in PGHD collection and using the data16,18,22,33.
Educational programmes for both HCPs and patients were identified as necessary to improve digital literacy15,26,29,30. For HCPs, this would enhance their ability to recommend and interact with PGHD tools, including data interpretation. For patients, these programmes could help to ensure correct usage and accurate data collection.
HCPs called for a clear regulatory framework addressing regulatory and ethical concerns such as data privacy and data security measures as well as third-party access to data19,30. Additionally, HCPs called for financial support to help patients from lower socioeconomic backgrounds to access PGHD tools31.
Evolving patient-provider roles in a changing system
Traditionally, the healthcare system has been dominated by paternalistic structures, with physicians being the primary decision-makers in patients’ care journeys. This is rooted in the belief that physicians possess superior medical knowledge and expertise, making them the most capable of judging what’s best for patients. However, in recent years, medicine has become gradually more participatory. This trend is supported by the emerging use of consumer health technologies that offer individuals unprecedented access to health information, health tracking features and personalised data insight reports. These advancements create opportunities for shared decision-making and a participatory approach to medicine, transforming the patient-provider relationship into a more equitable, empowered dynamic. HCPs in numerous studies in this review noted the potential of apps and wearables for collaboration on personal health goals and jointly deciding on PGHD to be integrated into care plans11,16,18,19,22,28,33,35,39,40. This approach effectively balances patients’ needs with clinical relevance while mitigating the risk of overburdening HCPs with excessive and unnecessary PGHD. However, acceptance of this approach varied depending on the clinical setting, highlighting the contrast between acute and paternalistic versus chronic and participatory medicine. For instance, physicians and cardiologists in two studies found limited utility of PGHD in acute or emergency care settings, where such data might impede timely care delivery24,40. Another study reported a lack of interest in PGHD from surgeons, whereas cardiologists found the data valuable and speculated that paternalistic structures may remain relevant in acute care settings but are outdated for long-term chronic disease management39. Given the increasing prevalence of chronic conditions and the subsequent rising demand for health services, engaging patients in proactive self-management will be crucial.
Some HCPs in chronic care20,37, acute, post-surgery care settings18 or not-specified care22 prefer to control which patients track what types of data to ensure medical relevance and integration into care plans, avoiding unnecessary workload18,20,22,37. They were holding on to traditional paternalistic thinking. Many HCPs also wanted only selected patients to share PGHD11,18,20,35,37. Suggested criteria included patients with memory issues35,37, poorly managed conditions11,37, patients at risk18, or with sufficient digital literacy20. HCPs emphasised the need to protect patients for whom an excessive focus on PGHD could be stressful19,22,29,30,39, particularly those predisposed to obsessive behaviour or mental disorders19,22,39. Some GPs expressed concerns about an emerging “entertainment medicine”, where healthy individuals who need it the least engage in excessive tracking and over-medicalisation of health40. Other GPs worried about “body estrangement” when measured data and bodily experiences do not match. Balancing these diverse perspectives is essential for the effective integration of PGHD into clinical practice30.
link