Developing an app-based self-management program for people living with HIV: a randomized controlled pilot study during the COVID-19 pandemic

Study design
A randomized controlled pilot trial was conducted to evaluate the self-management program’s effectiveness. The study protocol was registered with the Clinical Research Information Service [Registration number KCT0004696, 04/02/2020].
Participants
PLWH in Korea were recruited using convenient sampling. Participants were enrolled in the infectious outpatient clinic of a single hospital by the researcher. The inclusion criteria were: being diagnosed with HIV, aged above 19 years, receiving ART, using an Android smartphone, and being able to read and respond to questionnaires using a smartphone. Exclusion criteria were receiving a medical diagnosis that required immediate treatment and counseling in addition to HIV diagnosis. The required sample size was estimated using G*power 3.1 software20. The sample size derived was 28 when the effect size was 0.25, the first type error was 5%, the power was 80%, the number of comparison groups was two, and the number of measurements was three. Considering a drop out in the final sample, we divided 33 participants into intervention and control groups. No adverse events were reported by any of the participants.
The enrollment and follow-up were conducted from April to June 2020. The researcher randomly assigned the participants to the intervention and control groups in a 1:1 ratio via the Research Randomizer platform21. The intervention group employed the app for 4 weeks, while the control was provided with self-management educational materials in a portable document format. The nature of the intervention prevented participant blinding.
Health manager mobile app intervention
This intervention was developed based on the information-motivation-behavioral skills (IMB) model22,23 (Fig. 1). The “Health Manager” mobile app had 13 menus: Home, My Info, Health Goals, Alarms, Health Diary, Education Video, Health Information Bulletin Board, Community, Health Counselling, Health Points, Survey, Settings, and Help. The participants engaged in the self-management program through this app for 4 weeks (Fig. 2).
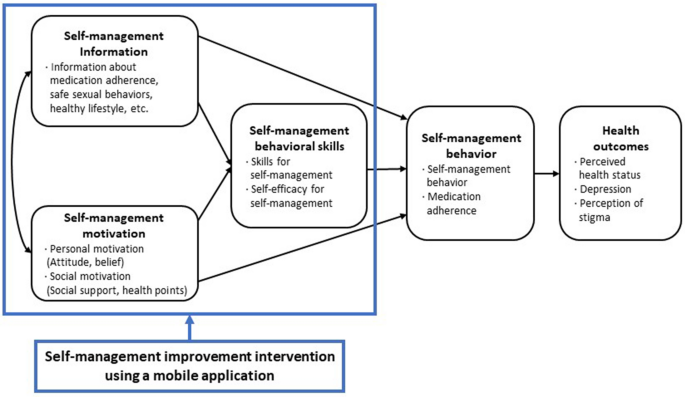
Conceptual framework of the self-management program using a mobile application.
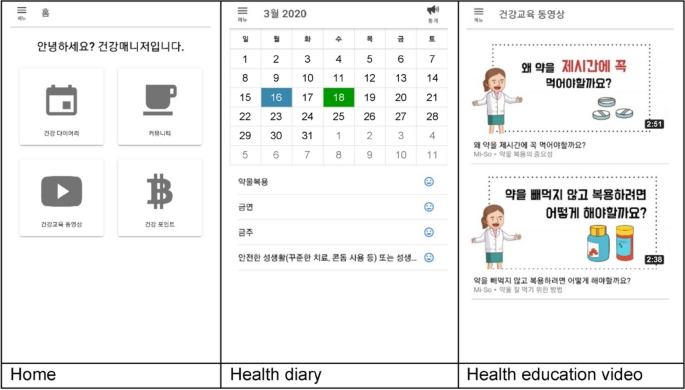
Screenshots of the mobile application’s main menu.
Intervention group participants installed the app on their mobile phones through the webpage as guided by the researcher. After user registration and the researcher’s approval process, they logged in to the mobile app. The participants were automatically shown the “Help” menu on the first login which informed them about the menus and the app’s usage. The last page of the “Help” menu was linked with the “Health goals” menu to enter the participants’ health information and goals for medication adherence, smoking, alcohol consumption, and sex life, and set a time for the medication alarm.
The self-management information was provided through the “Educational videos” and “Health information bulletin board” menus. Nine videos on medication adherence, smoking, alcohol consumption, and sex life were included, that could be watched per convenience; on the bulletin board, the researcher updated news about HIV and the health care information on mental health and strengthening immunity once or twice weekly. To improve self-management motivation, the “Community,” “Health counseling,” and “Health points” menus were used. The “Community” menu comprised three bulletin boards to share health care tips, worries, and their own stories freely with other PLWH. Participants could post at any time and communicate through replies. In the “Health counseling” menu, one-on-one counseling with nurses was provided through the bulletin board. Additionally, in the “Health points” menu, the participants could check the points accumulated through community activities (posts and replies), diary writing, and surveys, and exchange them for online gift vouchers. Through the “Health goals,” “Alarms,” and “Health diary” menus, the participants learned and employed behavioral skills for self-management by setting objectives regarding medication adherence, smoking, alcohol consumption, and sex life; setting reminders for medications (daily) and receiving encouragements to achieve their health goals (thrice a week); and recording their medication schedule and self-management behaviors.
Measurement
Primary outcomes
The primary outcomes of this study were self-efficacy for self-management, self-management behaviors, and medication adherence, and these variables related to self-management outcomes. Self-efficacy for self-management was assessed using the eight items developed by Wallston et al., translated into Korean by the researchers with the authors’ permission24. Each item was rated on a six-point Likert scale (1–6); the number of points corresponded to the level of self-efficacy. Items one, two, six, and seven were calculated inversely. This scale’s Cronbach’s α was 0.78 and 0.65 in Wallston et al.’s study24 and this research, respectively.
Self-management behaviors of PLWH were assessed using Webel et al.’s25 scale, translated into Korean by Kim et al.26. Subsequently, Kim et al. added four questions27 after in-depth interviews with PLWH. Each item was rated on a four-point Likert scale (0–3); the number of points corresponds to the level of the self-management behaviors. This scale’s Cronbach’s α was 0.87 and 0.82 in Kim et al.’s study27 and this research, respectively.
The self-reported questionnaire about medication adherence, proposed by Simoni et al., was adapted for the Korean context to evaluate consultation services for PLWH in hospitals by the Korea Disease Control and Prevention Agency (KCDA)28. The original scale included 5 questions regarding forgetting to take medication, 24 regarding why medication was not taken, 1 about the rate at which the respondent had taken medication during the past month, and questions about CD4 + T cell counts and blood viral load. The questionnaire in this study had six questions: five regarding forgetting to take medication and one about the rate at which the respondent had properly taken medication during the past month; the latter item was used to analyze the intervention’s effect.
Secondary outcomes
The secondary outcomes of this study were perceived health status, depression, and perceived stigma of HIV. Perceived health status was measured using a five-point Likert scale (very bad, bad, moderate, good, and very good) for the question “What is your overall health status currently?”.
The Korean version of the Patient Health Questionnaire-9 developed by Kroenke et al. was used to measure depression29. This scale was provided for free by Pfizer. Each item was rated on a four-point Likert scale (0–3); the scores were correlated with the level of depression. This scale’s Cronbach’s α was 0.87 for PLWH in Choi et al.’s study30 and 0.93 in this research.
Perceived stigma regarding HIV was determined using six items from the scale developed by Kalichman et al.31. Positive and negative responses were assigned “1” and “0” points, respectively. The total score corresponded to the perceived degree of stigma. This scale’s Cronbach’s α was 0.75 and 0.73 in Kalichman et al.’s study31 and in this research, respectively.
Satisfaction with the program (intervention group only)
Program satisfaction was scored using a 10-point Likert scale. Five questions were related to the acquisition of self-management information, change in participants’ emotional states (such as anxiety and depression), improvement in their communication with the nurses, specific behavioral skills they had acquired, and enhancement of their social support. One question addressed the program’s necessity to help PLWH improve their self-management behaviors. Further, participants were asked open-ended questions regarding aspects of the app that were positive, and those that could be improved.
Disease and health-related characteristics
Participants responded to 11 health-related questions, including on the time of HIV diagnosis, source of health information, self-help group participation, smoking habits, alcohol consumption, exercise frequency, weekly breakfast schedule, night sleep duration, and sleep quality on a four-point Likert scale (very bad, bad, good, and very good).
Furthermore, they reported the date of their most recent blood test, and the viral load and CD4 + T cell counts were determined using a self-report questionnaire with three items. The responses were verified through the hospital’s electronic medical records after receiving the Institutional Review Board’s (IRB) approval and participants’ consent.
Demographic characteristics
Participants were requested to report their age, sex, marital status, education level, working status, type of residence, income, and sexual identity.
Data collection
All participants were informed of the research purpose, content, and methods, and that their participation was voluntary, they could withdraw at any point, and their data would be kept confidential. All participants were requested to provide their informed consent online prior to participating in the study, and responded to the questionnaire only if they consented.
Both groups completed online self-report surveys using mobile phones. The data were collected thrice: immediately before the participants received their respective interventions; 4 weeks later, that is, immediately after the intervention ended; and 4 weeks after the intervention terminated, that is, 8 weeks after it began. During the second round, the intervention group was asked about how satisfied they were with the app. Gifticons ($10 per survey, total $30) were provided as an incentive for study participation.
Statistical analysis
First, a descriptive analysis of the primary and secondary outcomes, disease and health-related characteristics, and demographic characteristics was performed. Then, a homogeneity test for dependent variables and characteristics of the intervention and control groups was conducted using the chi-square test, Fisher’s exact test, independent t-test, and Wilcoxon signed rank test. The comparison of primary and secondary outcome variables between the intervention and control group was conducted using the generalized estimating equation (GEE), a statistical method that can analyze repeated measures data; it helps assess non-normally distributed outcomes that frequently occur in actual clinical fields32. Here, covariates in the GEE analysis included the variables with significant differences between the groups in the homogeneity test results; further, the correlation structure was set as exchangeable. Additionally, the intention-to-treat analyses were performed. Finally, the differences between dropouts and participants were analyzed using the chi-square test, Fisher’s exact test, independent t-test, and Wilcoxon signed rank test. The data were analyzed using SPSS v25.0 (IBM Corp., Armonk, NY, USA) and Stata/SE 15.0 (StataCorp, TX, USA).
Ethics approval
This study was approved by the IRB of the Yonsei University Health System (IRB Approval number: 4-2019-0884). The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate
The IRB of the Yonsei University Health System did not require the participants to provide written consent to evaluate the program’s effectiveness, given the study’s low risk and the fact that the intervention was provided through the Internet. All participants were informed of the research purpose, content, and methods, and that their participation was voluntary, they could withdraw at any point, and their data would be kept confidential. Before they responded to the survey, they were provided explanations of the study purpose and procedures. Moreover, they were able to respond to the questionnaire only upon agreeing to participate in the study.
link







