Evaluating Usability of and Satisfaction with mHealth app in Rural and Remote Areas -Germany GIZ Collaboration in Bosnia-Herzegovina to Optimize Type 1 Diabetes Care
1 Introduction
Type 1 diabetes mellitus (T1DM), a common chronic condition in children and adolescents, requires lifelong insulin therapy, regular blood glucose monitoring, diabetes education, and collaborative care to achieve favorable treatment outcomes (1, 2). Personalized holistic care with frequent monitoring and adjustments of insulin doses has been recommended (3), with optimal adherence to the devised treatment plan, along with adequate self-management activities, being the cornerstone of effective T1DM management (4). Suboptimal adherence to insulin regimens, as well as to food and exercise recommendations, is common in pediatric patients with T1DM, resulting in poor glycemic control (5–8), increased morbidity, and premature mortality (9). Suboptimal diabetes control during childhood can lead to the development of late diabetes complications when these patients reach adulthood (5, 10). The costs of diabetes complications are significant and represent a substantial economic burden on healthcare systems worldwide (11–14).
Although the incidence of T1DM in children and adolescents is highly variable, this incidence and the global burden of T1DM is increasing worldwide (1, 15, 16). In 2022, a total of 8.75 million people were living with T1DM worldwide, with 1.9 million living in low- and lower-middle-income countries, including 1.52 million aged <20 years, accounting for 182,000 deaths in 2022 (17). According to the International Diabetes Federation (IDF), the highest number of patients with T1DM in 2022 lived in Europe, followed by North America and the Caribbean regions (17). The highest incidence of T1DM in upper-middle-income countries were in Europe and Brazil (1). The World Health Organization (WHO) has reported that the prevalence of diabetes in Bosnia-Herzegovina (BiH) was 9.3%, being one of the highest rates in Europe (17). The annual incidence of T1DM per 100,000 inhabitants under age 18 years was 7.5 in the peripheral parts of BiH from 1998 to 2010 and 6.5 in the central region of BiH from 1999 to 2004 (18). Unofficial data estimate that the number of newly diagnosed patients has doubled in the last 10 years. The unreported numbers are likely much higher, as BiH does not yet have a nationwide registry for T1DM.
The everyday management of T1DM significantly burdens the affected patients and their families in BiH (19). Healthcare resources are insufficient to cope with the growing demand, with resources in BiH remaining below the regional average for universal health coverage owing to many factors, including the limited-service capacity and poor access among the most disadvantaged members of the population (20). There are only five diabetes reference centers nationwide, with only six specialist pediatric diabetologists, who practice in the major urban areas, Sarajevo, Tuzla, Zenica, Mostar, and Banja Luka (21). BiH, however, remains one of the most rural countries in Europe, with around 60% of the population living in rural areas (22). In rural and remote areas of BiH, children with T1DM are diagnosed and provided with insulin therapy by healthcare centers and ambulances (21). A structured diabetes training provided by an interdisciplinary diabetes team, which is highly essential for controlling the disease according to International Society for Pediatric and Adolescent Diabetes (ISPAD) guidelines (2), is often unavailable. Children with diabetes-related complications are transferred to the closest diabetes reference centers, which are often at full capacity and cannot take on additional patients. The COVID-19 pandemic worsened the situation for these patients. One study from BiH reported that treatment of diabetes complications had a major share in overall diabetes management costs (18).
The American Diabetes Association (ADA) has recommended regular follow-up, ongoing nutrition and diabetes self-management education (DSME) and support (DSMS), and access to specialized healthcare as necessary for effective T1DM management (23). Technology-based interventions can be helpful in patients who miss appointments and/or have poor accessibility to specialized T1DM care (10, 24), as these interventions enable remote monitoring and increase access to evidence-based practices outside conventional clinical settings (25, 26). The use of diabetes technology has increased markedly among children and adolescents with T1DM worldwide (27), with guidelines recommending that it be integrated into pediatric diabetes care (2, 28). The ADA has classified diabetes technology into three categories: hardware, devices, and software, which help patients manage their diabetes (29). mHealth interventions have been recommended for improving diabetes management among young people (30, 31). One such example is the increasing use of diabetes mobile apps to support disease management, prevent diabetes-related complications, and improve overall quality of life (32, 33). Diabetes apps have been reported effective in improving clinical outcomes and diabetes self-management (31, 34–37). The functions of diabetes apps developed to date have been found to vary, as various apps have focused on blood glucose monitoring, self-management, motivation for medication adherence, and lifestyle modifications (38, 39). Tailored education, timely feedback (26), remote monitoring, and follow-up (39) can enable diabetes apps to reduce the risks of increased HbA1c levels, psychosocial problems, and the development of complications associated with disrupted clinic visits (30). Moreover, diabetes apps have great potential for T1DM management in children and adolescents owing to the widespread, viability, and acceptance of the use of technology among young persons (24, 40, 41).
The development of diabetes apps and evidence supporting their efficacy and effectiveness in T1DM management (24, 40, 42–45) suggest that the use of these apps can effectively extend patient-centered care to remote and rural areas of BiH with limited access to specialized diabetes care, as well as providing frequent points of contact with specialist pediatric diabetologists. Evaluations of mHealth interventions in different contexts can influence their implementation, such as the settings in which they are used, the HCPs, and the entire implementation process (46). Accordingly, the current study was designed to assess the initial usability of a mHealth intervention among children and adolescents with T1DM, as well as their parents and HCPs, after using the Diabetes: M app for three months. User satisfaction, experience, and the perceived usefulness of various mHealth app features were investigated using a questionnaire. Findings of this study may provide reference points for the usability of the mHealth app in T1DM management from the perspectives of pediatric patients, their parents, and HCPs in underserved and remote areas.
2 Materials and methods
2.1 Study design and data collection
This cross-sectional survey conducted in February–March 2023 collected data related to user satisfaction, experience, and the usefulness of different features of the Diabetes: M app in managing T1DM. Pediatric patients with T1DM, along with their parents and HCPs, who had been enrolled in the 3-month mHealth-based T1DM management program, trained and connected through a digital health network, were invited to participate in the present survey. This T1DM management program established a digital network that included all children, adolescents, and young adults with T1DM across Middle Bosnia Canton in Bosnia-Herzegovina. All participants were approached and recruited through their respective diabetes type 1 patient organizations in Bugojno and Vitez. The network utilized a mobile health app and remote digital monitoring to bridge the gap between the main pediatric diabetology clinic in Sarajevo and the remote regions of Middle Bosnia Canton. The network also included local pediatricians and doctors from the hospital in Bugojno, Middle Bosnia Canton, Additionally, an online Viber peer support chat group was integrated into the network, facilitating daily communication and exchange among patients and healthcare providers. The establishment and complete structure of the digital health network has been described elsewhere (47).
Patients with T1DM were divided into two age groups, children aged ≤12 years, along with their parents, and adolescents/young adults aged >12 years. This division was based on the rationale that parents of children aged ≤12 years are generally responsible for managing T1DM in their children, whereas adolescents/young adults during the period of transition of care generally require less support from their parents and start managing their disease on their own (40, 42). Three online versions of the survey (i.e., for children/parents, adolescents/young adults, and HCPs) were created using Qualtrics XM®. The links, which were generated separately, were distributed through email and WhatsApp groups to the enrolled T1DM pediatric patients/parents and HCPs. Data were collected within 3 months after the end of the mHealth-based T1DM management program to minimize recall bias. The questionnaires were kept short to reduce respondent fatigue.
2.2 mHealth app intervention
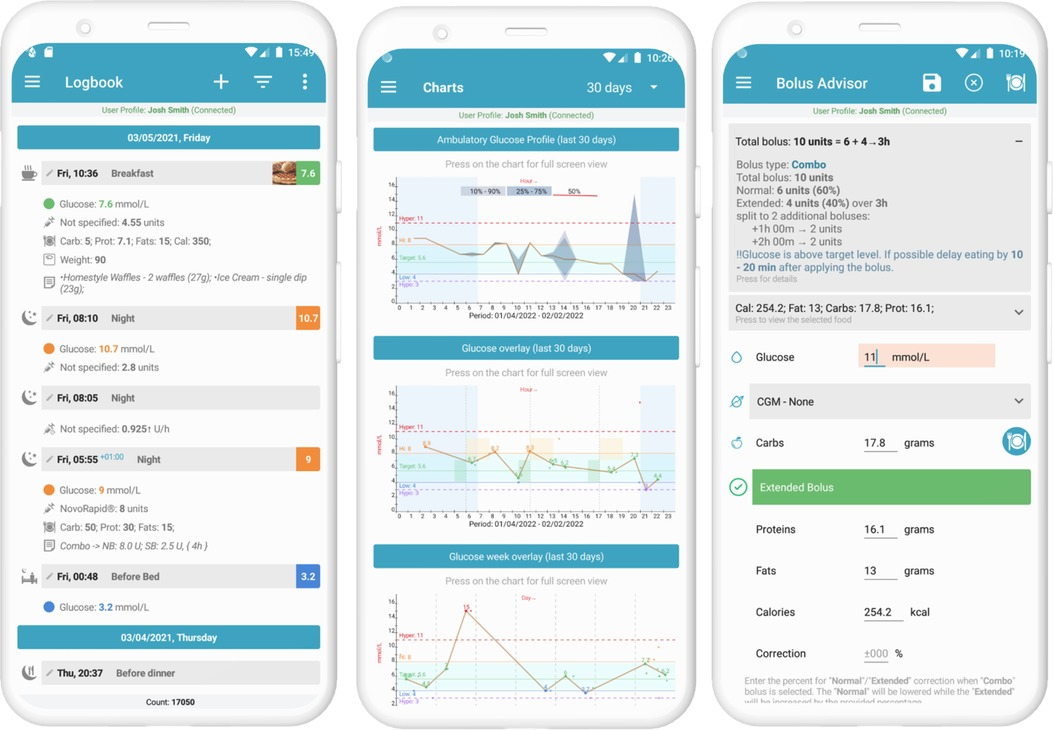
The Diabetes: M app platform consists of a mobile app (Figure 1) and a clinician monitoring system (Figures 2, 3) developed by Sirma Medical Systems. This app has been shown useful in improving diabetes control in T1DM patients (48) and is used in managing and monitoring all types of patients with diabetes and pre-diabetes. Table 1 shows a detailed description of its key features.
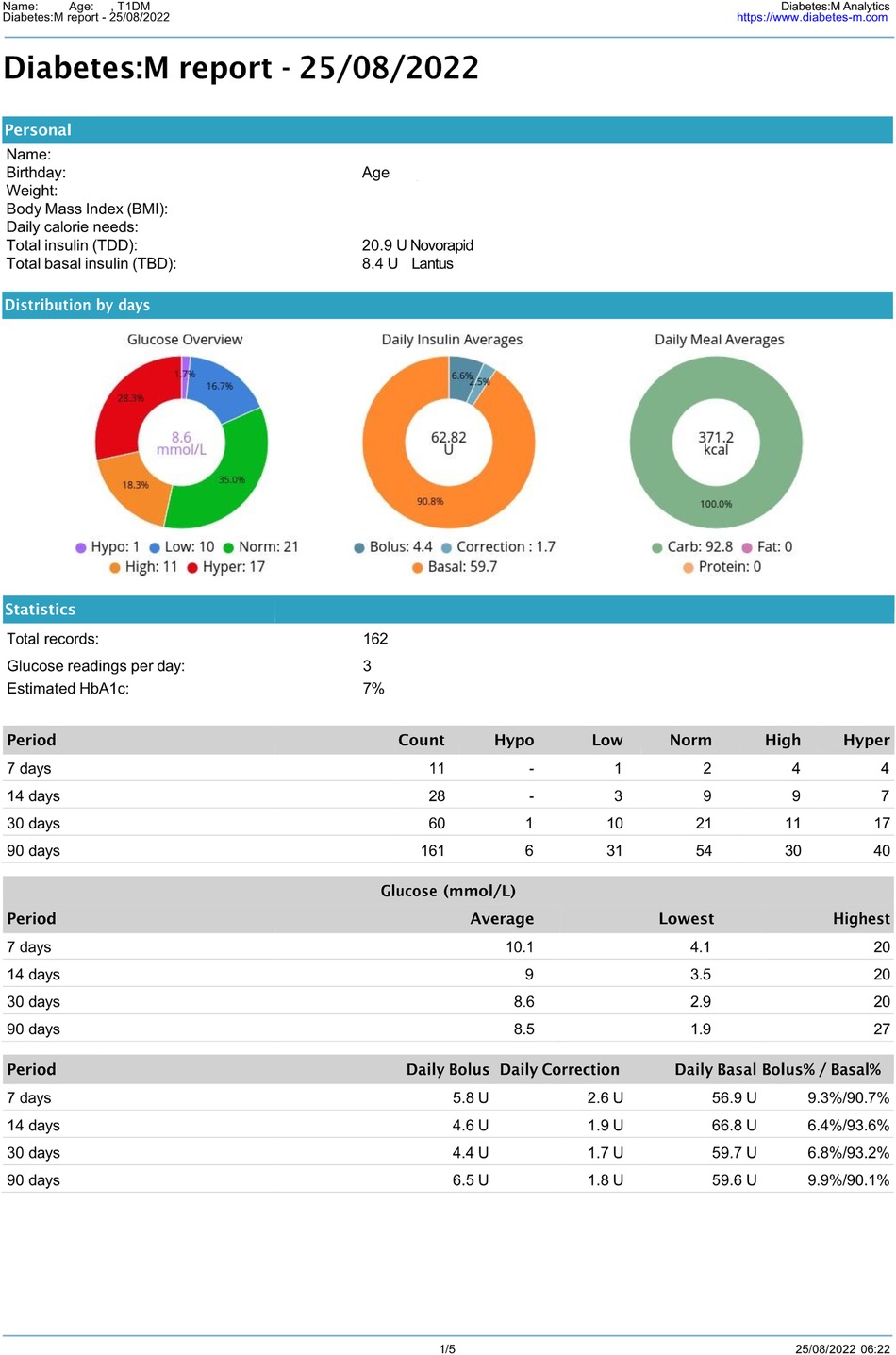
Figure 3 Anonymous 14-year-old T1DM patient’s Diabetes: M report. (1st page only).
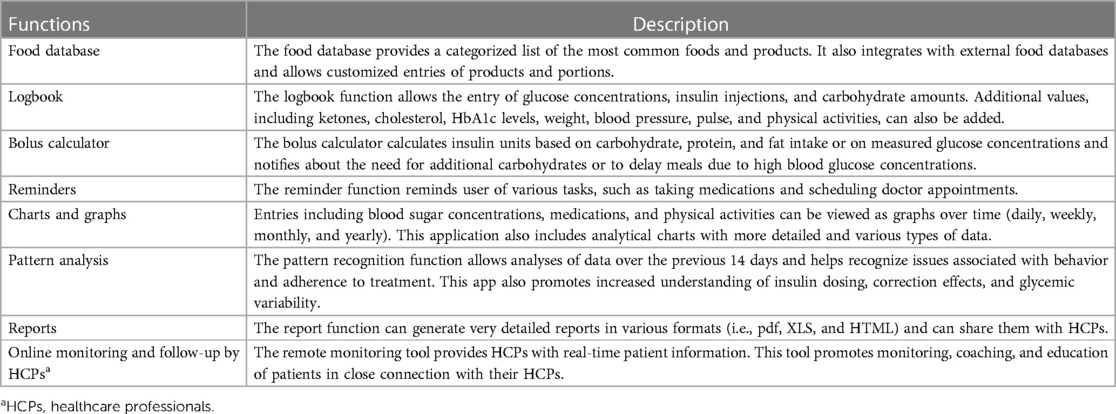
Table 1 Key features of the diabetes: M app, an interactive mHealth app consisting of a smartphone application for patients and a remote monitor software platform for HCPs.
2.3 Outcome measures
The three questionnaires, for T1DM children/parents, adolescents/young adults, and HCPs, were each divided into four main parts: participant characteristics, satisfaction and ease of use, the usefulness of different app features, and user experience with the app in managing T1DM. The questionnaires were developed in English, then translated into Bosnian by forward and back translation methods and adapted for cultural considerations. The two experts in pediatric diabetology checked these questionnaires for face and content validity. Based on their feedback, each questionnaire was revised to its final form. Participant information sheets were also evaluated for comprehensibility and appropriateness.
2.3.1 Participant characteristics
The first part of the questionnaires included questions about the sociodemographic characteristics of the participants, including age, gender, previous experience with mHealth apps, professional experience, and healthcare specialty (in the HCP questionnaire only).
2.3.2 Satisfaction and ease of use
The primary objective of the usability element was to assess patient and provider satisfaction and ease of use of the app. This objective was measured using the 8-item subscale of the Mobile Health App Usability Questionnaire (MAUQ) (49), a reliable and validated questionnaire with four different versions depending on the type of app (interactive or standalone) and the target user of the app (patient or provider). The MAUQ Interactive app, provider, and patient versions were selected for this study. Satisfaction and ease of use were measured using a 7-point Likert-type scale, ranging from 1 (strongly disagree) to 7 (strongly agree). A higher score indicated greater satisfaction with and ease of use of the app.
2.3.3 Usefulness of different app features
Assessments of the app usability included evaluations of the usefulness of different app features, namely logbook, food database, reminders, bolus calculator, charts and graphs, reports, pattern analysis, and online monitoring by HCPs (8 items). Each item was graded on a 5-point Likert-type scale, ranging from 1 (not at all useful) to 5 (extremely useful). A higher score indicated greater perceived usefulness of the app feature. Participants were also provided with the option “I have not used this feature.”
2.3.4 User experience with the app
The fourth part of the questionnaires consisted of questions examining participants’ experiences with the app in the management of T1DM. These questions, based on previous studies (24, 49, 50), were adjusted depending on the populations being assessed i.e., pediatric patients, parents, and providers. All of these questions were dichotomous, although participants could also state that they were uncertain.
2.4 Data analysis
Data were extracted from QualtricsXM® and descriptive statistics were analyzed using Microsoft Excel (2019). Categorical variables were reported as frequencies and percentages, whereas continuous variables were reported as means, standard deviations (SD), and ranges.
2.5 Ethical considerations
The current study is part of the project “Improving Diabetes Type 1 Care in Children and Adolescents in Bosnia and Herzegovina” (51) and was approved by the Ethics Committee of the Medical Association of the Central Bosnian Canton (Ethical Approval number 839/22). Subjects were provided with a detailed participant information sheet and informed consent/assent documents, with all participants providing written informed consent/assent using the checkbox option before starting the online survey. The individual questions were not linked and could be skipped to continue the questionnaire. The anonymity of the participants was ensured at all times, and the study was conducted in accordance with the Declaration of Helsinki (52).
3 Results
3.1 Outcome measures
3.1.1 Participant characteristics
From August to September 2022, 50 children, adolescents, and young adults diagnosed with T1DM were screened and enrolled in a 3-month mHealth-based T1DM management program. In addition, nine HCPs were trained in the use of the mHealth app in diabetes management as part of the digital health network (47). All 50 T1DM patients (children, adolescents, and young adults) and all nine HCPs completed the program and responded to the online survey, leading to response rates of 100% The mean ± SD age of T1DM patients was 14 ± 4.54 years (Table 2). Thirty-five (70%) were aged >12 years, 26 (52%) were female, and 37 (74%) reported previous experience with mHealth apps. The mean ± SD age of the HCPs was 43.4 ± 7.76 years; all nine (100%) were women and had a mean ± SD professional experience of 17.8 ± 8.81 years. Five (56%) of the nine HCPs had no previous experience using mHealth apps professionally. The nine HCPs included seven physicians, one pharmacist, and one other healthcare professional.
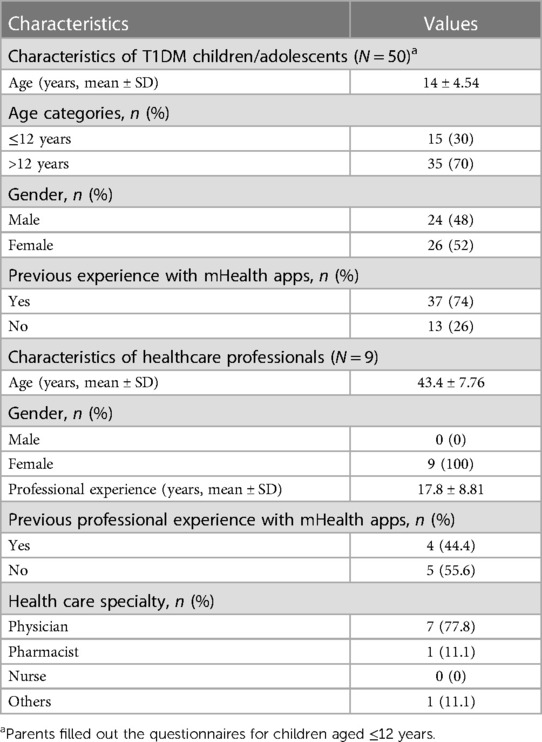
Table 2 Demographic characteristics of the participants included in this cross-sectional study.
3.1.2 Satisfaction and ease of use
Satisfaction with the mHealth app intervention was determined by measuring eight items on the MAUQ subscale for satisfaction and ease of use. The app was reported usable in the domains of ease-of-use and satisfaction by the T1DM children/parents (5.82/7.0), T1DM adolescents/young adults (5.68/7.0), and HCPs (5.22/7.0), indicating that all participants rated the Diabetes: M app as satisfactory (Table 3). The agreement among T1DM pediatric patients/parents for satisfaction and ease of use was >80% (Figures 4A, 4B), whereas the agreement among HCPs was 74.5% (Figure 4C).
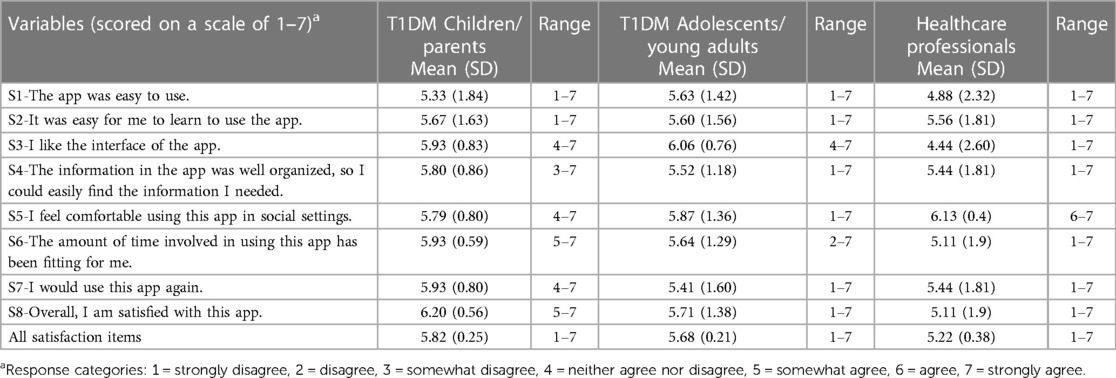
Table 3 Satisfaction of the participants with the diabetes: M app, measured using the mHealth App usability questionnaire (MAUQ) sub-scale for satisfaction and ease of use.
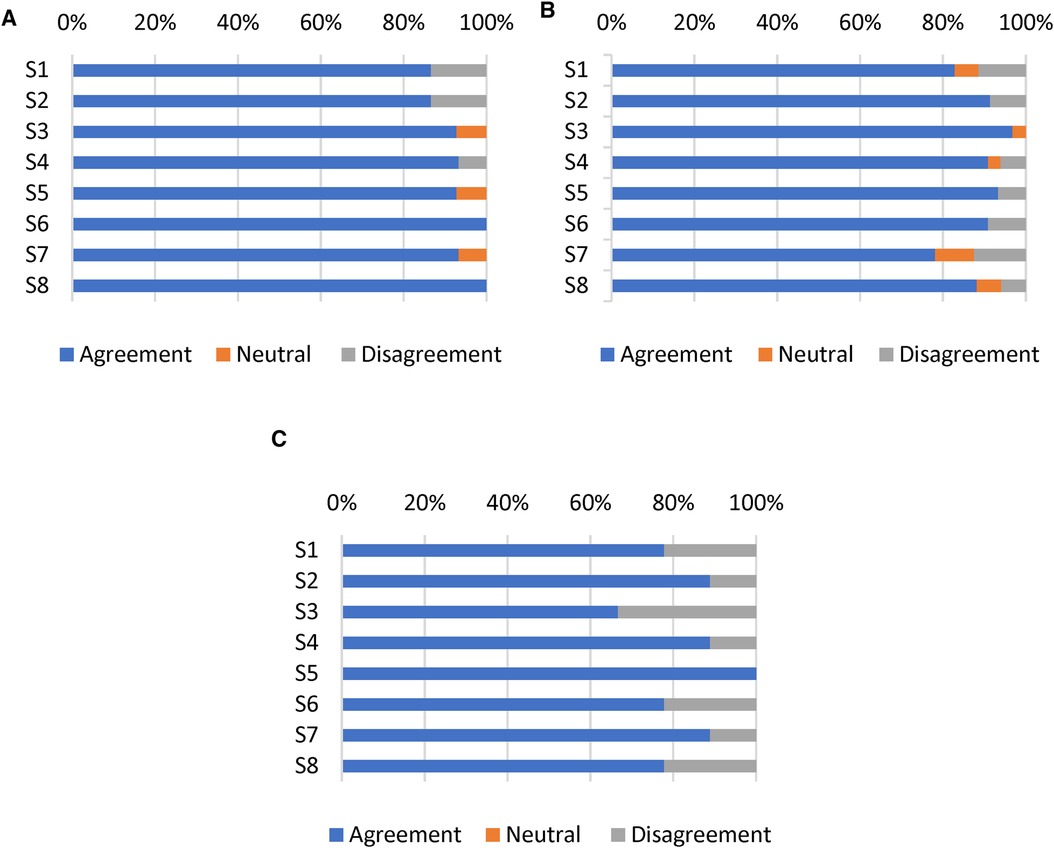
Figure 4 Responses of (A) T1DM children/parents, (B) T1DM adolescents/young adults, and (C) HCPs to eight items on the satisfaction and ease of use subscale of the MAUQ. Results reported as the percentages of participants who achieved scores on a 7-point Likert scale (Strongly agree, agree, and somewhat agree defined as agreement; strongly disagree, disagree, and somewhat disagree defined as disagreement).
3.1.3 Usefulness of app features
The mean usefulness score was more than 3 for all 8 features in both patient age groups. The top three key app features rated by T1DM children/parents who used these features during the 3-month trial of the T1DM management program were the reports (3.93/5), bolus calculator (3.91/5), and online monitoring and follow-up by HCPs (3.90/5) (Figure 5A). Similarly, T1DM adolescents and young adults found online monitoring and follow-up by HCPs (3.87/5), bolus calculator (3.79/5), and charts and graphs (3.65/5) to be more useful (Figure 5B). The HCPs rated bolus calculator (4.42/5), pattern analysis (4.3/5), and reports (4.3/5) to be the top three “extremely useful/very useful” app features for T1DM management with a mean usefulness score of more than 4 for all 8 features (Figure 5C).
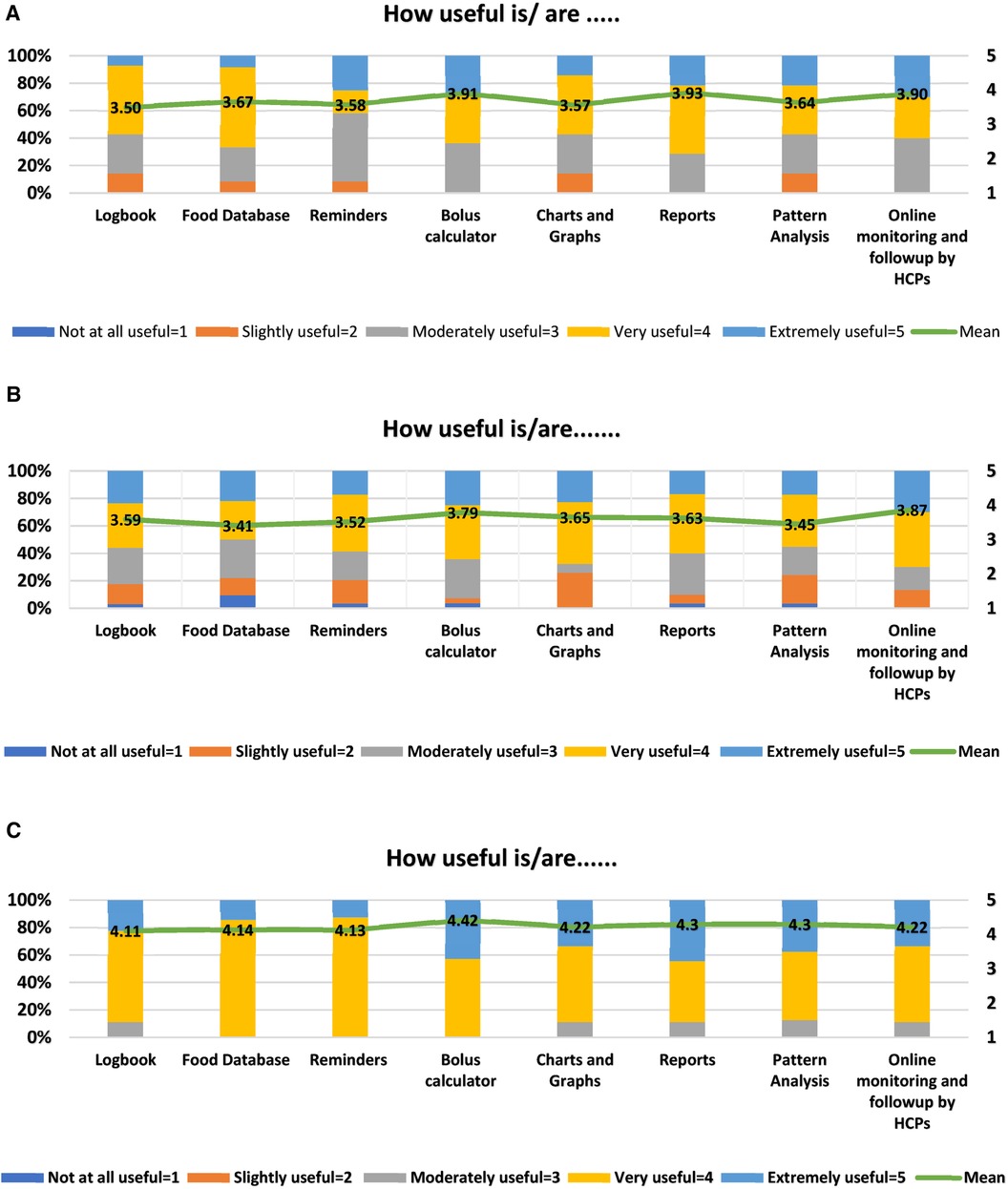
Figure 5 (A) diabetes: M app features rated by children/parents (n = 15). Mean values are shown in the bars. (B) Diabetes: M app features rated by adolescents/young adults (n = 35). Mean values are shown in the bars. (C) Diabetes: M app features rated by HCPs (n = 09). Mean values are shown in the bars.
3.1.4 User experience with the app
The nine HCPs rated their experiences with the Diabetes: M app in managing and supporting T1DM care of their pediatric patients as highly favorable. Only two of the nine HCPs encountered technical issues, with six reporting improvements in managing their patients with diabetes. Eight of the HCPs reported that use of the app improved their communications with patients, their understanding of patients’ diabetes management, and their sharing of information and other educational material (Table 4).
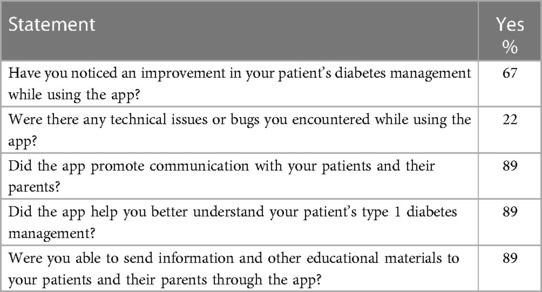
Table 4 HCPs’ experience with the app (N = 9).
Of the 15 parents evaluated, seven (47%) noticed improvements in their child’s T1DM management while using the app. Only four (27%) noticed changes in their child’s daily activities, with two (13%) encountering technical problems while using the app. Eight (54%) parents agreed that the app helped them communicate with HCPs, and ten (67%) found that use of the app provided better understanding of their child’s T1DM management (Table 5).

Table 5 Parents’ experience with the app (N = 15).
Of the 35 adolescents/young adults, 15 (43%) noticed improvements in their diabetes management, whereas only six (17%) encountered technical issues while using the app. Sixteen (46%) of the 35 adolescents/young adults agreed that the app helped them communicate better with their HCPs. Nineteen (54%) adolescents/young adults found that the app enabled better management of their T1DM while traveling or during unexpected events, with Twenty-two (63%) being better able to understand their diabetes management (Table 6).

Table 6 Adolescents’/young adults’ experience with the app (N = 35).
4 Discussion
This study provides insights into the initial usability of the mHealth app intervention among T1DM pediatric patients/parents and their HCPs. Our findings provide clues to the management of T1DM pediatric patients with limited access to healthcare facilities, especially to patients living in rural and remote areas. The survey items focused on the subjective evaluations by T1DM patients and their parents and by HCPs of satisfaction, ease of use, usefulness, and experience with the mHealth app intervention following the completion of a 3-month mHealth-based T1DM management program. At the end of the 3-month trial, the participants expressed satisfaction with the Diabetes: M app, with children/parents having an overall mean ± SD satisfaction score of 5.82 ± 0.25, adolescents/young adults with an overall mean ± SD satisfaction score of 5.68 ± 0.21, and HCPs having an overall mean ± SD satisfaction score of 5.22 ± 0.38, each on a 7-point Likert-type scale. Moreover, HCPs had a largely positive view of the usefulness of different app features and rated the app as more efficient than T1DM pediatric patients and their parents.
Patient satisfaction, defined as outcomes meeting patient expectations (53), has been associated with greater compliance to devised treatment plans and more improved outcomes (54). Satisfaction with patient-centered mHealth technologies is usually assessed using descriptive and quantitative measurements (53). The high mean overall satisfaction scores among T1DM pediatric patients and their parents in the present study indicate high satisfaction and ease of use in engaging with the mHealth app. This is in line with previous studies showing high levels of satisfaction with mHealth apps among children and adolescents with T1DM, as well as their parents (24, 42, 43, 55–58).
As revealed by different items on the MAUQ multi-item sub-scale (49), T1DM pediatric patients and their parents reported that the app was easy to use and easy to learn to use. These participants liked the app interface and found that the information component of the app was well-organized and easily accessible. They felt comfortable using the app in social settings, similar to findings showing that adolescents found that mHealth apps were an acceptable method of communicating with their parents, particularly in social settings (42). Participants’ satisfaction in the present study was also shown by their intention to use the app again, similar to previous results showing that 96% of study subjects indicated intent to use the app if available outside the trial (43). “The amount of time involved in using this app has been fitting for me” was also rated positively by the respondents, as was the overall satisfaction with the app. These high scores for satisfaction and ease of use may have been due to the ease of use of the simple platform and the familiarity of younger patients with mHealth apps, as 74% reported previous use of mHealth apps. High patient satisfaction in our study may also be due to the prompt responses of HCPs to their patients via an online platform created specifically for this purpose. Moreover, the nine HCPs who took part in the study also rated the app as satisfactory. In other studies, HCPs have also reported high satisfaction with mHealth-based systems for chronic disease management in children and adolescents (24, 59).
Participants were also asked to rate the usefulness of different app features in assisting with daily T1DM self-management. Overall, pediatric patients and their parents found various app features to be “moderately useful to very useful,” whereas HCPs rated these app features to be “very useful to extremely useful.” Online monitoring and follow-up by HCPs, bolus calculator, and reports were the top three rated features by the T1DM children/parents. Similarly, adolescents and young adults found online monitoring and follow-up by HCPs, bolus calculator, and charts and graphs to be more useful. This contrasts with previous studies conducted in New Zealand and Canada where the logbook feature was among the most favored and used features (60, 61). We found clues for this finding in the local context of Bosnia and Herzegovina where the Diabetes: M app could not be integrated with continuous glucose monitoring (CGM) systems and the patients had to enter their blood glucose levels manually every time. This may have led to possible patient fatigue and less interest in blood glucose tracking feature and consequent usability. However, similar to our study a Scottish survey reported the blood glucose data feature was felt useful by a minority of patients and the bolus calculator was the most desired feature by T1DM patients (62). Connected through the digital health network, the patients were regularly monitored by the HCPs which is depicted by the favorable rating of online monitoring and follow-up feature by the patients and their parents. During the 3 month T1DM management program, the HCPs responded to immediate patient needs. Nevertheless, the frequency of remote monitoring and communication consistency can be decided mutually among HCPs and patients/parents that fit their daily activities. Moreover, scaling up such mHealth interventions requires an adequate number of dedicated well-trained HCPs, an appropriate workload, task shifting, and other encouragement approaches such as monetary incentives and opportunities for training (63–66). In our study, HCPs rated the bolus calculator, reports, and pattern analysis as the most highly useful features. Graphically and statistically generated reports and pattern analysis were rated as helpful by HCPs in another study of children and adolescents with T1DM (24).
Although HCPs were very positive about their experience with the Diabetes: M app in managing T1DM, pediatric patients and their parents rated their experience as moderate. For further implementation, it is therefore important to highlight all stakeholders’ lived initial user experiences and insights. These findings about the optimal functional experience are also important for the long-term engagement of users and sustained use of apps beyond the initial adoption stage owing to the problem of high dropout rates and less user retention (67).
Although the results of the present study correspond with those of earlier studies, the present findings indicate that the mHealth solutions might fill the care gaps and compensate for a lack of functional health infrastructure in remote and rural areas with limited to almost no facilities. Patients in rural areas with limited healthcare access can get an advantage from digitally assisted remote care options, however, existing care should be supplemented gradually instead of substitution, keeping in mind the local context and individual patient characteristics (68). The favorable attitude of HCPs and T1DM pediatric patients/parents towards the mHealth app underlines their great interest and has led to increased adoption of mHealth care services by these populations (69, 70). Increased satisfaction with diabetes apps provides evidence for the increased implementation of mHealth interventions for the management of chronic diseases. Launching the diabetes program in BiH was not easy due to complexities in the political and economic situation in BiH and large regional disparities in diabetes care of children and adolescents. Unless they live in larger cities with pediatric diabetology clinics, these children do not have regular access to pediatric diabetes clinics or pediatric diabetologists. Children and their families often have to self-manage this complex and life-long disease, with late complications becoming inevitable. One of the key successes of this initiative was the active participation of patients and providers, with response rates of 100%. Moreover, this initiative included active parent involvement, improving T1DM management among children (71, 72), particularly through the mHealth apps (73). After enrollment into the digital health network, participants received formal training on how to use the mHealth app based on the significance of adequate training for using and implementing mHealth interventions (46, 74). This digital network can serve as an example for other remote or rural regions and it can accommodate other chronic disease management apps.
The present study had several limitations, including its use of self-reported measures, with these data subject to recall bias, possibly skewing the results. Another limitation was the small sample size, however, we had a minimal proportion of missing data. A third limitation was the short period of mHealth app usage of only 3 months. Finally, The current study may contain a response bias as all the HCPs, and T1DM patients/their parents voluntarily participated in the T1DM management program and subsequent survey, and might therefore be more enthusiastic and positive about the mHealth app or more motivated to use the app. Studies that include a larger number of patients and a longer period of mHealth-based T1DM management are needed to provide more robust conclusions.
5 Conclusion
A mobile app intervention, involving communications between pediatric patients with T1DM living in remote and rural areas of BiH and large specialized pediatric diabetology clinics in the capital city or other cities can facilitate diabetes care for the former. This app and other mHealth apps can be useful in overcoming the limitations of diabetes care in rural areas and improving diabetes management and HCP-patient communications. The positive experiences and satisfaction with the app reported by patients, their parents, and HCPs may be useful for other HCPs and policymakers in BiH and other countries with similar circumstances, suggesting that mHealth apps can facilitate the delivery of healthcare services. Randomized controlled trials with objective clinical outcomes, such as HbA1c and other glucose biomarkers, are needed to determine the efficacy and sustainability of mHealth interventions in pediatric patients with T1DM.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the Medical Association of the Central Bosnian Canton. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
BAS: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SL: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing. SH: Project administration, Writing – review & editing. EO: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The project was funded through GIZ (German Development Cooperation), Clinical Partnership, Project number: 81277521.
Acknowledgments
The authors would like to thank GIZ Clinic Partnerships for funding the project. The authors would like to thank the participants for participating in the study, and for completing the evaluation questionnaires. We thank Professor Reinhard Holl, Institute of Epidemiology and Medical Biometry, ZIBMT, University of Ulm, Germany for his valuable input and project education and support. The research work of BAS was supported by a Scholarship from the Higher Education Commission (HEC), Pakistan in collaboration with the German Academic Exchange Service (DAAD), Germany.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ogle GD, James S, Dabelea D, Pihoker C, Svennson J, Maniam J, et al. Global estimates of incidence of type 1 diabetes in children and adolescents: results from the international diabetes federation atlas, 10th edition. Diabetes Res Clin Pract. (2022) 183:109083. doi: 10.1016/j.diabres.2021.109083
PubMed Abstract | Crossref Full Text | Google Scholar
2. Limbert C, Tinti D, Malik F, Kosteria I, Messer L, Jalaludin MY, et al. ISPAD clinical practice consensus guidelines 2022: the delivery of ambulatory diabetes care to children and adolescents with diabetes. Pediatr Diabetes. (2022) 23:1243–69. doi: 10.1111/pedi.13417
PubMed Abstract | Crossref Full Text | Google Scholar
3. Ziegler R, Neu A. Diabetes in childhood and adolescence. Dtsch Ärztebl Int. (2018) 115:146–56. doi: 10.3238/arztebl.2018.0146
Crossref Full Text | Google Scholar
4. Hood KK, Rohan JM, Peterson CM, Drotar D. Interventions with adherence-promoting components in pediatric type 1 diabetes: meta-analysis of their impact on glycemic control. Diabetes Care. (2010) 33:1658–64. doi: 10.2337/dc09-2268
PubMed Abstract | Crossref Full Text | Google Scholar
5. Gandhi K, Vu BK, Eshtehardi SS, Wasserman RM, Hilliard ME. Adherence in adolescents with type 1 diabetes: strategies and considerations for assessment in research and practice. Diabetes Manag (Lond). (2015) 5:485–98. doi: 10.2217/dmt.15.41
PubMed Abstract | Crossref Full Text | Google Scholar
6. Borus JS, Laffel L. Adherence challenges in the management of type 1 diabetes in adolescents: prevention and intervention. Curr Opin Pediatr. (2010) 22:405–11. doi: 10.1097/MOP.0b013e32833a46a7
PubMed Abstract | Crossref Full Text | Google Scholar
7. Patino AM, Sanchez J, Eidson M, Delamater AM. Health beliefs and regimen adherence in minority adolescents with type 1 diabetes. J Pediatr Psychol. (2005) 30:503–12. doi: 10.1093/jpepsy/jsi075
PubMed Abstract | Crossref Full Text | Google Scholar
8. Schober E, Wagner G, Berger G, Gerber D, Mengl M, Sonnenstatter S, et al. Prevalence of intentional under- and overdosing of insulin in children and adolescents with type 1 diabetes. Pediatr Diabetes. (2011) 12:627–31. doi: 10.1111/j.1399-5448.2011.00759.x
PubMed Abstract | Crossref Full Text | Google Scholar
9. Harjutsalo V, Forsblom C, Groop PH. Time trends in mortality in patients with type 1 diabetes: nationwide population based cohort study. Br Med J. (2011) 343:d5364. doi: 10.1136/bmj.d5364
Crossref Full Text | Google Scholar
10. Prahalad P, Tanenbaum M, Hood K, Maahs DM. Diabetes technology: improving care, improving patient-reported outcomes and preventing complications in young people with type 1 diabetes. Diabet Med. (2018) 35:419–29. doi: 10.1111/dme.13588
PubMed Abstract | Crossref Full Text | Google Scholar
11. Hex N, Bartlett C, Wright D, Taylor M, Varley DJDM. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med. (2012) 29:855–62. doi: 10.1111/j.1464-5491.2012.03698.x
PubMed Abstract | Crossref Full Text | Google Scholar
12. Yang W, Cintina I, Hoerger T, Neuwahl SJ, Shao H, Laxy M, et al. Estimating costs of diabetes complications in people <65 years in the U.S. using panel data. J Diabetes Complications. (2020) 34:107735. doi: 10.1016/j.jdiacomp.2020.107735
PubMed Abstract | Crossref Full Text | Google Scholar
13. Andersson E, Persson S, Hallén N, Ericsson Å, Thielke D, Lindgren P, et al. Costs of diabetes complications: hospital-based care and absence from work for 392,200 people with type 2 diabetes and matched control participants in Sweden. Diabetologia. (2020) 63:2582–94. doi: 10.1007/s00125-020-05277-3
PubMed Abstract | Crossref Full Text | Google Scholar
14. Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Bärnighausen T, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care. (2018) 41:963–70. doi: 10.2337/dc17-1962
PubMed Abstract | Crossref Full Text | Google Scholar
15. Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Hosseini Fard HH, Ghojazadeh M. Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis. Health Promot Perspect. (2020) 10:98–115. doi: 10.34172/hpp.2020.18
PubMed Abstract | Crossref Full Text | Google Scholar
16. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. (2014) 103:137–49. doi: 10.1016/j.diabres.2013.11.002
PubMed Abstract | Crossref Full Text | Google Scholar
18. Catic T, Popovic SP, Asimi ZV, Hlavinkova L. Costs of diabetes mellitus and its complications in Bosnia and Herzegovina. Mater Sociomed. (2022) 34:149–54. doi: 10.5455/msm.2022.34.149-156
PubMed Abstract | Crossref Full Text | Google Scholar
23. Chiang JL, Kirkman MS, Laffel LM, Peters AL. Type 1 diabetes sourcebook authors. Type 1 diabetes through the life span: a position statement of the American diabetes association. Diabetes Care. (2014) 37:2034–54. doi: 10.2337/dc14-1140
PubMed Abstract | Crossref Full Text | Google Scholar
24. Berndt RD, Takenga C, Preik P, Kuehn S, Berndt L, Mayer H, et al. Impact of information technology on the therapy of type-1 diabetes: a case study of children and adolescents in Germany. J Pers Med. (2014) 4:200–17. doi: 10.3390/jpm4020200
PubMed Abstract | Crossref Full Text | Google Scholar
25. Leonardsen AL, Hardeland C, Helgesen AK, Grøndahl VA. Patient experiences with technology enabled care across healthcare settings- a systematic review. BMC Health Serv Res. (2020) 20:779. doi: 10.1186/s12913-020-05633-4
PubMed Abstract | Crossref Full Text | Google Scholar
26. Knox EC, Quirk H, Glazebrook C, Randell T, Blake H. Impact of technology-based interventions for children and young people with type 1 diabetes on key diabetes self-management behaviours and prerequisites: a systematic review. BMC Endocr Disord. (2019) 19:1. doi: 10.1186/s12902-018-0327-2
PubMed Abstract | Crossref Full Text | Google Scholar
27. Auzanneau M, Karges B, Neu A, Kapellen T, Wudy SA, Grasemann C, et al. Use of insulin pump therapy is associated with reduced hospital-days in the long-term: a real-world study of 48,756 pediatric patients with type 1 diabetes. Eur J Pediatr. (2021) 180:597–606. doi: 10.1007/s00431-020-03883-2
PubMed Abstract | Crossref Full Text | Google Scholar
28. Kumar VS, Wentzell KJ, Mikkelsen T, Pentland A, Laffel LM, The D. The DAILY (daily automated intensive log for youth) trial: a wireless, portable system to improve adherence and glycemic control in youth with diabetes. Diabetes Technol Ther. (2004) 6:445–53. doi: 10.1089/1520915041705893
PubMed Abstract | Crossref Full Text | Google Scholar
30. Mayor S. Use texts, apps, and skype to keep young people with diabetes engaged with services, says guidance. Br Med J. (2016) 352:i394. doi: 10.1136/bmj.i394
Crossref Full Text | Google Scholar
31. Wang X, Shu W, Du J, Du M, Wang P, Xue M, et al. Mobile health in the management of type 1 diabetes: a systematic review and meta-analysis. BMC Endocr Disord. (2019) 19:21. doi: 10.1186/s12902-019-0347-6
PubMed Abstract | Crossref Full Text | Google Scholar
34. Hou C, Xu Q, Diao S, Hewitt J, Li J, Carter B. Mobile phone applications and self-management of diabetes: a systematic review with meta-analysis, meta-regression of 21 randomized trials and GRADE. Diabetes Obes Metab. (2018) 20:2009–13. doi: 10.1111/dom.13307
PubMed Abstract | Crossref Full Text | Google Scholar
35. Eberle C, Löhnert M, Stichling S. Effectiveness of disease-specific mhealth apps in patients with diabetes mellitus: scoping review. JMIR MHealth UHealth. (2021) 9:e23477. doi: 10.2196/23477
PubMed Abstract | Crossref Full Text | Google Scholar
36. Whitehead L, Seaton P. The effectiveness of self-management mobile phone and tablet apps in long-term condition management: a systematic review. J Med Internet Res. (2016) 18:e97. doi: 10.2196/jmir.4883
PubMed Abstract | Crossref Full Text | Google Scholar
37. Bonoto BC, de Araújo VE, Godói IP, de Lemos LL, Godman B, Bennie M, et al. Efficacy of mobile apps to support the care of patients with diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. JMIR MHealth UHealth. (2017) 5:e4. doi: 10.2196/mhealth.6309
PubMed Abstract | Crossref Full Text | Google Scholar
38. Ali Sherazi B, Laeer S, Krutisch S, Dabidian A, Schlottau S, Obarcanin E. Functions of mhealth diabetes apps that enable the provision of pharmaceutical care: criteria development and evaluation of popular apps. Int J Environ Res Public Health. (2022) 20:64. doi: 10.3390/ijerph20010064
PubMed Abstract | Crossref Full Text | Google Scholar
39. Ye Q, Khan U, Boren SA, Simoes EJ, Kim MS. An analysis of diabetes mobile applications features compared to AADE7™: addressing self-management behaviors in people with diabetes. J Diabetes Sci Technol. (2018) 12:808–16. doi: 10.1177/1932296818754907
PubMed Abstract | Crossref Full Text | Google Scholar
40. Barnes TL, Lee S, Thompson N, Mullen K, Chatterton P, Gandrud L. Barriers to glucose testing and attitudes toward mobile app and device use in a large cohort of T1D pediatric patients: implications for diabetes management. J Diabetes Sci Technol. (2018) 12:1246–7. doi: 10.1177/1932296818794706
PubMed Abstract | Crossref Full Text | Google Scholar
41. Vaala SE, Hood KK, Laffel L, Kumah-Crystal YA, Lybarger CK, Mulvaney SA. Use of commonly available technologies for diabetes information and self-management among adolescents with type 1 diabetes and their parents: a web-based survey study. Interact J Med Res. (2015) 4:e24. doi: 10.2196/ijmr.4504
PubMed Abstract | Crossref Full Text | Google Scholar
42. Holtz B, Mitchell KM, Holmstrom AJ, Cotten SR, Dunneback JK, Jimenez-Vega J, et al. An mhealth-based intervention for adolescents with type 1 diabetes and their parents: pilot feasibility and efficacy single-arm study. JMIR mHealth UHealth. (2021) 9:e23916. doi: 10.2196/23916
PubMed Abstract | Crossref Full Text | Google Scholar
43. Goyal S, Nunn CA, Rotondi M, Couperthwaite AB, Reiser S, Simone A, et al. A mobile app for the self-management of type 1 diabetes among adolescents: a randomized controlled trial. JMIR mHealth UHealth. (2017) 5:e82. doi: 10.2196/mhealth.7336
PubMed Abstract | Crossref Full Text | Google Scholar
44. Kirwan M, Vandelanotte C, Fenning A, Duncan MJ. Diabetes self-management smartphone application for adults with type 1 diabetes: randomized controlled trial. J Med Internet Res. (2013) 15:e235. doi: 10.2196/jmir.2588
PubMed Abstract | Crossref Full Text | Google Scholar
45. Ryan EA, Holland J, Stroulia E, Bazelli B, Babwik SA, Li H, et al. Improved A1C levels in type 1 diabetes with smartphone app use. Can J Diabetes. (2017) 41:33–40. doi: 10.1016/j.jcjd.2016.06.001
PubMed Abstract | Crossref Full Text | Google Scholar
46. Ross J, Stevenson F, Lau R, Murray E. Factors that influence the implementation of e-health: a systematic review of systematic reviews (an update). Implement Sci. (2016) 11:146. doi: 10.1186/s13012-016-0510-7
PubMed Abstract | Crossref Full Text | Google Scholar
47. Emina O, Snijezana H, Elma R, Bushra AS, Florian K, Stephanie L. Mhealth clinic partnership project between HHU düsseldorf and bosnia-Herzegovina: creation of a digital network of diabetes type 1 children and adolescents in rural and remote areas in Bosnia and Herzegovina. ESDPPP Congress; Prague, Czech Republic (2023). p. 1. Available online at:
48. Korbut AI, Myakina N, Bulumbaeva DM, Fazullina ON, Koroleva EA, Klimontov VV. Reducing of glycemic variability in patients with type 1 diabetes by automated calculation of bolus insulin doses using Mobile devices. Proceedings of the 2019 International Multi-Conference on Engineering, Computer and Information Sciences (SIBIRCON); 21–27 October 2019; Novosibirsk, Russia (2019). p. 372–5
49. Zhou L, Bao J, Setiawan IMA, Saptono A, Parmanto B. The mhealth app usability questionnaire (MAUQ): development and validation study. JMIR mHealth UHealth. (2019) 7:e11500. doi: 10.2196/11500
PubMed Abstract | Crossref Full Text | Google Scholar
50. Carroll AE, DiMeglio LA, Stein S, Marrero DG. Using a cell phone-based glucose monitoring system for adolescent diabetes management. Diabetes Educ. (2011) 37(1):59–66. doi: 10.1177/0145721710387163
PubMed Abstract | Crossref Full Text | Google Scholar
52. General Assembly of the World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. (2014) 81(3):14–8.25951678
PubMed Abstract | Google Scholar
53. Bruce C, Harrison P, Giammattei C, Desai SN, Sol JR, Jones S, et al. Evaluating patient-centered mobile health technologies: definitions, methodologies, and outcomes. JMIR MHealth UHealth. (2020) 8:e17577. doi: 10.2196/17577
PubMed Abstract | Crossref Full Text | Google Scholar
55. Mulvaney SA, Anders S, Smith AK, Pittel EJ, Johnson KB. A pilot test of a tailored mobile and web-based diabetes messaging system for adolescents. J Telemed Telecare. (2012) 18:115–8. doi: 10.1258/jtt.2011.111006
PubMed Abstract | Crossref Full Text | Google Scholar
56. Chiang YT, Chang CW, Yu HY, Tsay PK, Lo FS, Chen CW, et al. Developing the “healthcare CEO app” for patients with type 1 diabetes transitioning from adolescence to young adulthood: a mixed-methods study. Nurs Open. (2023) 10:1755–66. doi: 10.1002/nop2.1432
PubMed Abstract | Crossref Full Text | Google Scholar
57. Cafazzo JA, Casselman M, Hamming N, Katzman DK, Palmert MR. Design of an mhealth app for the self-management of adolescent type 1 diabetes: a pilot study J. J Med Internet Res. (2012) 14:e70. doi: 10.2196/jmir.2058
PubMed Abstract | Crossref Full Text | Google Scholar
58. Knight BA, McIntyre HD, Hickman IJ, Noud M. Qualitative assessment of user experiences of a novel smart phone application designed to support flexible intensive insulin therapy in type 1 diabetes. BMC Med Inform Decis Mak. (2016) 16:119. doi: 10.1186/s12911-016-0356-6
PubMed Abstract | Crossref Full Text | Google Scholar
59. Kosse RC, Bouvy ML, de Vries TW, Koster ES. Evaluation of a mobile health intervention to support asthma self-management and adherence in the pharmacy. Int J Clin Pharm. (2019) 41:452–9. doi: 10.1007/s11096-019-00798-3
PubMed Abstract | Crossref Full Text | Google Scholar
60. Boyle L, Grainger R, Hall RM, Krebs JD. Use of and beliefs about Mobile phone apps for diabetes self-management: surveys of people in a hospital diabetes clinic and diabetes health professionals in New Zealand. JMIR Mhealth Uhealth. (2017) 5(6):e85. doi: 10.2196/mhealth.7263
PubMed Abstract | Crossref Full Text | Google Scholar
61. Dobson KG, Hall P. A pilot study examining patient attitudes and intentions to adopt assistive technologies into type 2 diabetes self-management. J Diabetes Sci Technol. (2015) 9(2):309–15. doi: 10.1177/1932296814560395
PubMed Abstract | Crossref Full Text | Google Scholar
62. Conway N, Campbell I, Forbes P, Cunningham S, Wake D. Mhealth applications for diabetes: user preference and implications for app development. Health Informatics J. (2016) 22(4):1111–20. doi: 10.1177/1460458215616265
PubMed Abstract | Crossref Full Text | Google Scholar
63. Gaudillère M, Pollin-Javon C, Brunot S, Villar Fimbel S, Thivolet C. Effects of remote care of patients with poorly controlled type 1 diabetes included in an experimental telemonitoring programme. Diabetes Metab. (2021) 47(6):101251. doi: 10.1016/j.diabet.2021.101251
Crossref Full Text | Google Scholar
64. Addotey-Delove M, Scott RE, Mars M. Healthcare Workers’ perspectives of mHealth adoption factors in the developing world: scoping review. Int J Environ Res Public Health. (2023) 20(2):1244. doi: 10.3390/ijerph20021244
PubMed Abstract | Crossref Full Text | Google Scholar
65. Brandt LR, Hidalgo L, Diez-Canseco F, Araya R, Mohr DC, Menezes PR, et al. Addressing depression comorbid with diabetes or hypertension in resource-poor settings: a qualitative study about user perception of a nurse-supported smartphone app in Peru. JMIR Ment Health. (2019) 6(6):e11701. doi: 10.2196/11701
PubMed Abstract | Crossref Full Text | Google Scholar
67. Deniz-Garcia A, Fabelo H, Rodriguez-Almeida AJ, Zamora-Zamorano G, Castro-Fernandez M, Alberiche Ruano MDP, et al. Quality, usability, and effectiveness of mHealth apps and the role of artificial intelligence: current scenario and challenges. J Med Internet Res. (2023) 25:e44030. doi: 10.2196/44030
PubMed Abstract | Crossref Full Text | Google Scholar
68. Currie M, Philip LJ, Roberts A. Attitudes towards the use and acceptance of eHealth technologies: a case study of older adults living with chronic pain and implications for rural healthcare. BMC Health Serv Res. (2015) 15:162. doi: 10.1186/s12913-015-0825-0
PubMed Abstract | Crossref Full Text | Google Scholar
69. Silva BM, Rodrigues JJ, de la Torre Díez I, López-Coronado M, Saleem K. Mobile-health: a review of current state in 2015. J Biomed Inform. (2015) 56:265–72. doi: 10.1016/j.jbi.2015.06.003
PubMed Abstract | Crossref Full Text | Google Scholar
70. Ventola CL. Mobile devices and apps for health care professionals: uses and benefits. P T. (2014) 39:356–64.24883008
PubMed Abstract | Google Scholar
71. King PS, Berg CA, Butner J, Butler JM, Wiebe DJ. Longitudinal trajectories of parental involvement in type 1 diabetes and adolescents’ adherence. Health Psychol. (2014) 33:424–32. doi: 10.1037/a0032804
PubMed Abstract | Crossref Full Text | Google Scholar
72. Temmen CD, Lu R, Gee BT, Chen Z, Nansel TR. Latent classifications of parental involvement in diabetes management for youth with type 1 diabetes: a randomized clinical trial. Pediatr Diabetes. (2022) 23:1133–42. doi: 10.1111/pedi.13397
PubMed Abstract | Crossref Full Text | Google Scholar
73. Holtz BE, Mitchell KM, Holmstrom AJ, Hershey DS, Cotten SR, Dunneback JK, et al. The effect of an mhealth intervention for adolescents with type 1 diabetes and their parents. J Telemed Telecare. (2022):1357633X221125835. doi: 10.1177/1357633X221125835
PubMed Abstract | Crossref Full Text | Google Scholar
74. Davies MJ, Kotadia A, Mughal H, Hannan A, Alqarni H. The attitudes of pharmacists, students and the general public on mhealth applications for medication adherence. Pharm Pract. (2015) 13:644. doi: 10.18549/PharmPract.2015.04.644
Crossref Full Text | Google Scholar
link